“The relative humidity of air is 60%”—this statement means.
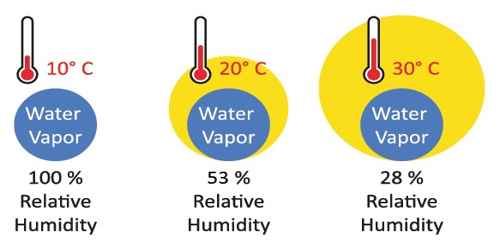
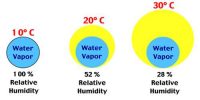
Relative humidity is defined as the ratio of the mass of water vapor present in a given volume of air at a particular temperature to the mass of water vapor required to saturate the same volume of air at the same temperature. That means relative humidity at a given temperature is the amount of water vapor in the air compared to how much water vapor air can hold at that temperature. It is the amount of water vapor (vapor pressure) that is in the air. It is a percentage of how much moisture the air could possibly hold.
(i) the vapor pressure at room temperature is 60/100 or 3/5 part of the saturated vapor pressure at the same temperature;
(ii) the water vapor present in a certain volume of air in the room is only 60% of the water vapor needed to saturate the air at room temperature.
Experiment- Spray some water inside a room and you will see that water vapor increases. But you will see that the temperature remains constant. Again, relative humidity and dew point will be increased. What is the meant?
When water is sprayed inside a room water vapor in air increases. But since temperature remains the same, hence the mass of water vapor needed to saturate the air inside the room at that temperature remains unchanged. In the definition of specific heat numerator increases and denominator decreases. Hence relative humidity increases. Obviously, the dew point also increases.
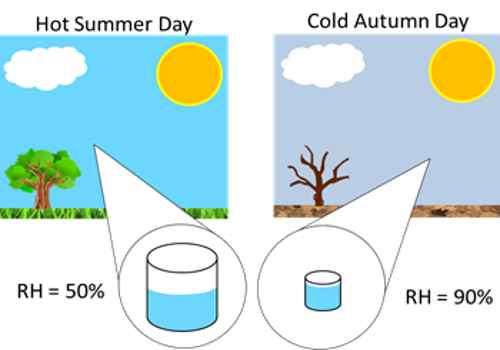
Here 60% Relative humidity means, air contains 60% of water vapor compared to how much it can hold at that temperature, if Relative Humidity is 100%, air no more can hold water vapor and drop them as showers. The relative humidity is the percent of saturation humidity, usually calculated in relation to saturated vapor density.
Relative Humidity = [Actual Vapor Density / Saturation Vapor Density] x 100 %.
The relative humidity is expressed by-
- partial vapor and air pressure,
- the density of the vapor and air, or
- by the actual mass of the vapor and air.
The relative humidity is a mixture utility of the real humidity substance of the air, the temperature, and the barometric pressure. Saturation occurs when the air is holding the utmost amount of water vapor probable at the existing pressure and temperature. Saturation is equal to 100% relative humidity, resulting in precipitation. The influence of relative humidity in our daily life is important which affects our physical and mental state. So we need to know the significance of relative humidity.
For example, Humans are very responsive to humidity, as the skin relies on the air to get rid of wetness. The procedure of sweating is your body’s endeavor to keep cool and preserve its present temperature. If the air is at 100 percent relative humidity, sweat will not disperse into the air. As a result, we experience much hotter than the real warmth when the relative humidity is high. If the relative humidity is low, we can feel much cooler than the real temperature because our sweat evaporates simply, cooling us off.