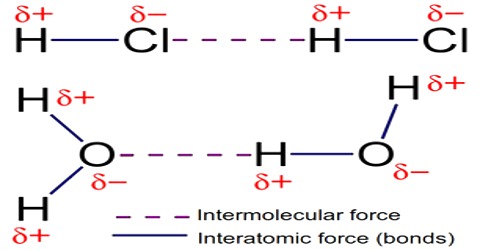
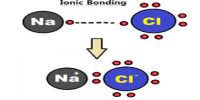
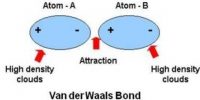
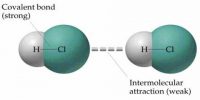
Interatomic Force in Solids
Solid body has fixed shape and volume. Due to strong attracting force particles remain very close to each other. Inter atomic attraction of solids is maximum and distance is minimum. By strong inter atomic attractive force particles remain tightly and solidly bound with each other. In this situation inter particle space is almost negligible. As the inter-particles remain in tightly bound condition by maximum attractive force, so it occupies a fixed position and a fixed volume Besides, as there is no sufficient space for motion particles do not change positions. Because of it, shape of the material is fixed. Although the particles in solid cannot change position, but can oscillate about their mean positions. These particles do not have translational or rotational motion. Van der Waals forces are the residual attractive or repulsive forces between molecules or atomic groups that do not begin from a covalent bond, or electrostatic interaction of ions or of ionic groups with one another or with impartial molecules.
As there is no vacant space between particles, so by applying pressure the volume of a solid body cannot contractual or be changed. This Inherent force is called inter-particle attractive force [Fig (a) and (b)]. The magnitude of this force depends on the distance between particles and distribution of particles. If the distance is large, the force becomes less, and closer the distance inter-particle force is large. But, if the distance becomes less than a particular value, repulsion between particles appear. All the tiny particles of matter above absolute zero temperature vibrate and acquire kinetic energies. Kinetic energy increases with the increase of temperature. If the inter-atomic force is larger than the kinetic energy of the particles, then the material remains in solid state. In this state, tiny particles cannot leave from their mean positions, only they create vibrations within themselves.