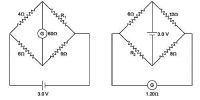
Daniel cell is a primary cell which cannot supply steady current for a long time. It consists of a copper vessel containing a strong solution of copper sulphate (Figure). A zinc rod is dipped in dilute sulphuric acid contained in a porous pot.
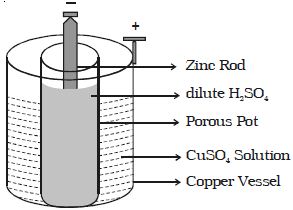
The porous pot is placed inside the copper sulphate solution. The zinc rod reacting with dilute sulphuric acid produces Zn++ ions and 2 electrons.
Zn++ ions pass through the pores of the porous pot and reacts with copper sulphate solution, producing Cu++ ions. The Cu++ ions deposit on the copper vessel. When Daniel cell is connected in a circuit, the two electrons on the zinc rod pass through the external circuit and reach the copper vessel thus neutralizing the copper ions. This constitutes an electric current from copper to zinc. Daniel cell produces an emf of 1.08 volt.