Chemical Structure of Hemoglobin (Hb)
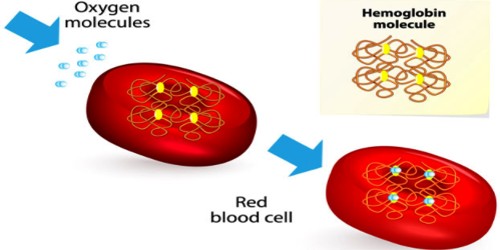
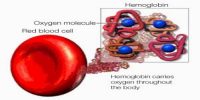
Hemoglobin (Hb) may be defined as a vital conjugated protein present inside the Red Blood Cells (RBC). It is the protein molecule in red blood cells that bear oxygen from the lungs to the body’s tissues and returns carbon dioxide from the tissues back to the lungs.
Hemoglobin consists of protein globin united with pigment heam. It is the oxygen-binding protein of red blood cells and is a globular protein with quaternary structure.
Heam: Heam is an iron-containing porphyrin known as iron porphyrin Ix. The porphyrin nucleus consists of 4 pyrole rings joined together by 4 methines (= CH -) bridges. The porphyrins are thus tetraphyroles. The pyrole rings are numbered i, ii, iii, and iv. Carbone atom of methine bridge is labeled α, β, δ, λ. The side chains 1, 3, 5, 8 are methyl 2.4 vinyl and 6,7 are propionic acid. Each heam group can carry one O2 molecule.
Globin: Global consists of 4 polypeptide subunits 2 subunits form α polypeptide chains and 2 subunits form β polypeptide chains. Each α chain contains 141 amino acid and β chains contain 146 amino adds.
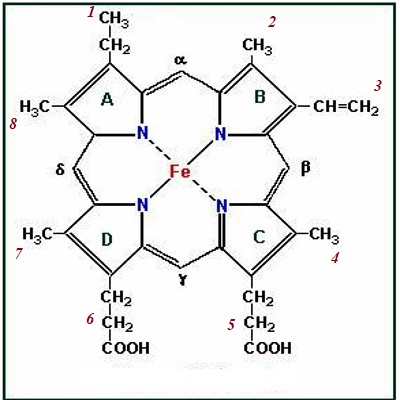
Fig: Chemical Structure of Hemoglobin
Polypeptide chain – Iron of heam is in the Ferrous form (Fe++). It is attached to the N of each pyrole ring and to the N of imidazole group in the associated globulin.