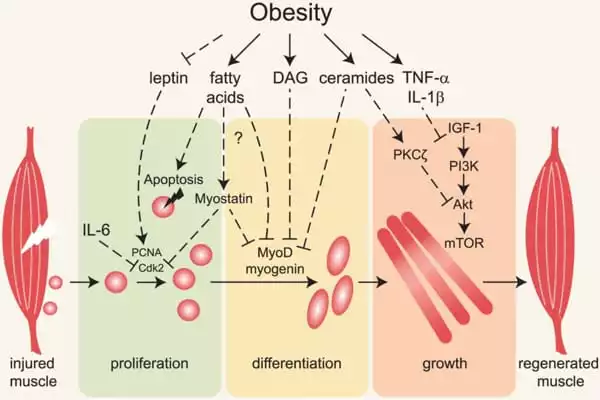
Obesity can impair skeletal muscle contractile function, reducing mobility and promoting obesity-related health risks. Obesity is linked to functional limitations in muscle performance as well as an increased risk of developing a functional disability such as mobility, strength, postural, and dynamic balance limitations. Reduced mobility, neural adaptations, and changes in muscle morphology may all contribute to this relative weakness.
Obese patients frequently experience a decrease in metabolism and skeletal muscle endurance, but the underlying mechanism is unknown. A research team led by Dr Chi Bun CHAN, Assistant Professor from the School of Biological Sciences, Faculty of Science, the University of Hong Kong (HKU), has discovered a new mechanism that explains how obesity impairs skeletal muscle function and offers a potential treatment for the disease. The findings of the study were recently published in the world-renowned scientific journal Autophagy.
Obesity is a metabolic disorder that is becoming more common in modern society. Obesity has tripled globally since the 1970s, reaching 650 million (13 percent of the total global population) in 2016. Obesity is well known to have negative effects on multiple human organs and to cause a variety of chronic diseases such as diabetes, hypertension, fatty liver disease, and atherosclerosis. Obese patients’ skeletal muscle fat metabolism is slower than that of healthy people, which scientists believe is due to abnormal mitochondrial functions (the powerhouses of a cell that convert nutrients into biological energy). However, how obesity affects mitochondrial activity is a long-unresolved question.
Clearly, muscle-derived BDNF is a weight-control protein because it increases energy expenditure while maintaining insulin sensitivity. Our research adds to the body of knowledge in this area, and we hope that by using our MBKO mice, we will be able to discover more functions of this myokine.
Dr. Chi Bun Chan
Dr. Chan’s team created a special obesified mouse model by removing the gene of brain-derived neurotrophic factor (BDNF) exclusively in their skeletal muscle to study the functional effects of obesity on the skeletal muscle. BDNF was discovered to be an important growth factor for the survival and activity of neurons. Recent research suggests that BDNF is a muscle-secreted protein (i.e., myokine), but its physiological significance is unknown.
Dr. Chan’s team discovered for the first time that obesity reduced the amount of BDNF in mouse skeletal muscle. They also discovered that mice lacking BDNF in their muscles, dubbed ‘MBKO’ (Muscle-specific BDNF Knockout), gained more body weight and developed severe insulin resistance when fed a high-fat diet. Furthermore, the researchers discovered that MBKO mice expend less energy than their control cohort.
The researchers used a variety of biochemical, histological, metabolomic, and molecular analyses to show that the mitochondria in the muscle of MBKO mice were unable to recycle, resulting in an accumulation of damaged mitochondria in the tissues. As a result, lipid metabolism in the muscle of MBKO mice was slowed, resulting in more lipid accumulation that interfered with insulin sensitivity.
“Clearly, muscle-derived BDNF is a weight-control protein because it increases energy expenditure while maintaining insulin sensitivity,” Dr. Chan said. “BDNF has long been thought to be a brain-specific peptide, and its importance in peripheral tissues has been overlooked. Our research adds to the body of knowledge in this area, and we hope that by using our MBKO mice, we will be able to discover more functions of this myokine” Dr. Chan continued.
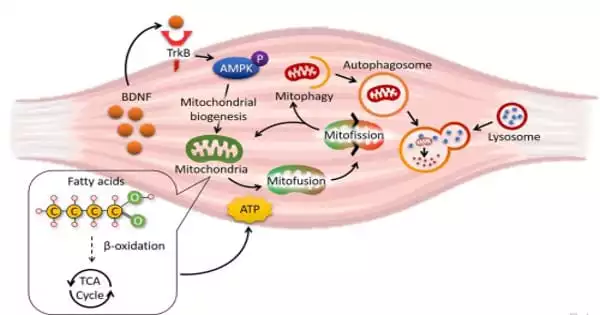
Dr. Chan’s team used cultured cell models in addition to animal studies to pinpoint the molecular mechanism for the defective mitochondrial turnover in BDNF-deficient muscle cells. They discovered that muscle-secreted BDNF used AMPK-activated protein kinase, a well-known energy sensor in cells, to activate the Parkin/PINK1 pathway in skeletal muscle, inducing mitophagy (a highly regulated mechanism to recycle materials in cells in response to various challenges).
To apply these findings to a therapeutic setting, the researchers investigated whether restoring BDNF signaling in muscle would reverse obesity-induced mitochondrial damage. They fed the obese mice 7,8-dihydroxyflavone, a natural bioavailable BDNF mimetic in plants (found in the leaves of Godmania aesculifolia, a South American plant species) that is currently being used in Alzheimer’s disease clinical trials, and discovered that obesity-induced mitochondrial dysfunction was alleviated.