Key components of synapse development, which is critical for neuronal communication and information storage in the brain, were revealed by researchers. They detected the creation of these vesicles in real neurons by using CRISPR to designate synaptic vesicles with a fluorescent protein in human stem cells.
Unlike popular perception, synaptic vesicle proteins, active zone proteins, and maybe adhesion proteins all share a uniform transport mechanism rather than distinct ones.
This groundbreaking discovery introduces the concept of a specialized neuron-specific transport organelle and discusses future implications for therapeutic interventions in neurological diseases and memory storage.
The researchers discovered that the proteins required for synapse formation, which were previously thought to travel individually, share a single ‘bus’ or transport pathway in neurons.

KIF1A, a kinesin motor protein, has been identified as a critical driver of axonal transport, and mutations in it are thought to affect axonal transport of pre-synaptic proteins, resulting in neurological difficulties such as movement disorders and mental incapacity.
Neuron-Specific Organelle Neurons may have a distinct transport organelle that lacks Golgi signs but shares markers with the endolysosomal system, an important discovery that could pave the way for understanding and potentially controlling neuronal regeneration and aging.
There is a sender and a recipient, as in every communication process: Axons are nerve cell processes that generate and transmit electrical signals, acting as signal senders.
Synapses are places of interaction between axonal nerve terminals (the pre-synapse) and synaptic neurons (the post-synapse). The electrical impulse is transformed at these synapses into chemical messengers, which are received and perceived by the neighboring neuron’s post-synapses. The messengers are released from synaptic vesicles, which are unique membrane sacs.
Synapses can store information in addition to transmitting it. While the structure and function of synapses are well established, little is known about their formation.
A team from Berlin’s Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) has now cast new light on this mystery. This astonishing breakthrough was also supported by scientists from Charité-Universitätsmedizin, the Max Delbrück Center for Molecular Medicine (MDC), and the Universities of Leipzig, Chicago, and Sheffield.
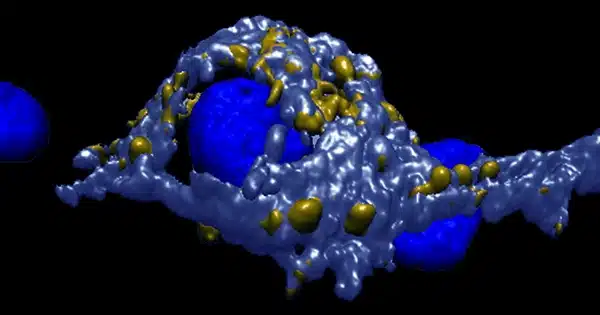
Fluorescent protein reveals the development of synaptic vesicles: The researchers employed CRISPR gene scissors to insert a fluorescent protein into human stem cells and then created neurons from the modified stem cells to track the creation of pre-synapses from the start. The fluorescent marker allowed the researchers to directly examine the emergence of nascent synaptic vesicles in living growing human nerve cells under the microscope.
The membrane vesicles that contain messengers and are stored at each synapse to transform electrical signals into chemical signals are known as synaptic vesicles. These vesicles compose the primary parts of the pre-synapse, together with scaffolding proteins that inform synaptic vesicles where the synapse is and calcium channels that chemically translate the electrical signal.
Because all three components have their own genes, they are made up of separate protein molecules. As a result, it was previously assumed that they also travel distinct paths before eventually coming together in one area to form a functional synapse.
All components set off together: However, the findings of the researchers contradict this idea. “The synaptic vesicle proteins and the proteins of the so-called ‘active zone,’ as well as likely the adhesion proteins that hold synapses together, share the same bus,” explains research group leader Professor Dr. Volker Haucke. “It was quite contentious. “However, our data in human neurons in culture appear to be quite clear.”
But how do the proteins reach the synapse-forming site? The researchers were able to demonstrate, for example, that axonal movement is powered by a mechanism of motor proteins in their investigation. Their findings point to a kinesin known as “KIF1A” as the primary driver. This motor protein is most well-known for its link to neurological diseases of the peripheral nervous system and the brain.
“We suspect that mutations in KIF1A interfere with axonal transport of pre-synaptic proteins, resulting in neurological symptoms such as movement disorders, ataxia, or mental disability,” Volker Haucke writes in the paper. The scientist is also a molecular pharmacology professor at Freie Universität Berlin.
Furthermore, the researchers were able to determine the axonal carriers’ cell-biological identification. This revealed another surprise: whereas the vast majority of secretory vesicles originate from the Golgi apparatus, axonal transport vesicles lack Golgi markers and instead share markers with the endolysosomal system, which is typically involved in the degradation of defective proteins in non-neuronal cells.
The researchers were able to define the size and form of the axonal transport vesicles using an innovative mix of light and high-resolution electron imaging.
Discovery of transport organelles that exist only in neurons: “Our findings suggest that neurons invented a new type of organelle, a transport organelle that may be unique to neurons,” said Dr. Sila Rizalar, FMP postdoctoral scholar and principal author of the study published in Science. “This was as little known as the shared transport pathway.”
Basic research findings may one day be valuable for clinical applications. After all, understanding the mechanism of axonal transport and the main proteins involved in order to intervene therapeutically is critical when the contact points between neurons fail, whether due to disease, injury, or the aging process.
“Ideally, it will be possible to restore or enhance axonal transport to promote neuronal regeneration or counteract aging,” according to Volker Haucke.
Although the researchers have uncovered a fundamental mechanism of synapse creation, many issues remain unsolved, including how the newly discovered transport organelles are generated, what they are made of, and how they convey their cargo – synapse molecules – to their destination.
It also raises the possibility that everlasting memories are stored via the same axonal transport mechanism that forms synapses. These are the questions Volker Haucke and his colleagues are eager to address. The possibilities are exciting.
Wherever there are nerve cells, there are synapses, whether in the brain or in the muscles. These neuronal contact sites serve as the foundation for excitation transmission, i.e. neuronal communication. There is a sender and a recipient, as in every communication process: Axons are nerve cell processes that generate and transmit electrical signals, acting as signal senders.
Synapses are places of interaction between axonal nerve terminals (the pre-synapse) and synaptic neurons (the post-synapse). The electrical impulse is transformed at these synapses into chemical messengers, which are received and perceived by the neighboring neuron’s post-synapses. The messengers are released from synaptic vesicles, which are unique membrane sacs.
Synapses can store information in addition to transmitting it. While the structure and function of synapses are well established, little is known about their formation.
A team from Berlin’s Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) has now cast new light on this mystery. This astonishing breakthrough was also supported by scientists from Charité-Universitätsmedizin, the Max Delbrück Center for Molecular Medicine (MDC), and the Universities of Leipzig, Chicago, and Sheffield.