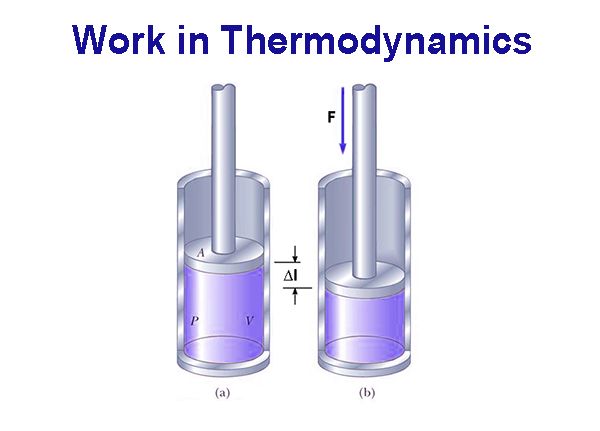
In thermodynamics, work has a broader meaning. It includes mechanical work, electrical work, surface work etc. Mechanical work (w) is said to be done when an object is moved a distance ∆Ι by an applied force F.
Accordingly,
w = F x ∆l
If as a result of the application of the force F the object moves an infinitesimally small distance dl then the infinitesimally small work done dw would be given by
dw = F x dl
Unit of work:
The unit of force in the CGS units is dyne and that of length is centimeter. Work in the same unit is expressed in ergs.
So, dyne × cm = erg; erg is the work done when a force of 1 dyne acts and causes of displacement of 1 cm.
Since an erg of work is a small quantity, a larger unite, joule, is often used- 1 joule = 107 erg.
Characteristics of heat and work:
(a) Both appear at the boundary of the system
(b) Both cause changes in the system
(c) Both produce equivalent and opposite effects in the surroundings.