Liquefaction of gases is the procedure by which substances in their gaseous state are converted to the liquid state. Liquefying a gas means the conversion of gas into a liquid.
There are two conditions for the liquefaction of gases. There are-
- By applying pressure
- By decreasing temperature
By Applying Pressure: At high pressure the volume of the gas decreases Therefore, the molecules of gas come close to each other due to which there is an intermolecular attraction between molecules. In this way, if we apply to define the amount of pressure then for some gases intermolecular attraction force reaches such an extent so that gas turns into liquid.
When pressure on a gas is increased, its molecules nearer together, and its temperature is condensed, which removes sufficient energy to make it transform from the gaseous to the liquid state.
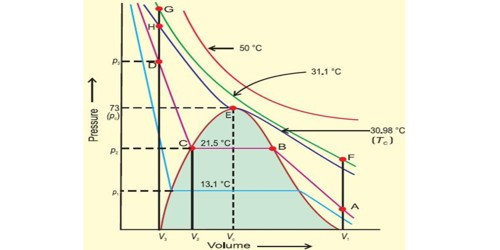
For example, ammonia has a critical temperature of 271°F (133°C). This temperature is well above room temperature. Thus, it is comparatively easy to convert ammonia gas to the liquid state just by applying adequate pressure. At its critical temperature, that pressure is 112.5 atmospheres.
By decreasing temperature: According to the kinetic theory of gases if the temperature is decreased kinetic energy of molecules also decreases and at the same time volume of gas also decreases. The molecules which come close to each other at low temperature cannot resist the intermolecular attraction of each other. Therefore the gas turns into liquid due to this attraction force. For example, Ammonia gas can be liquefied by applying high pressure and lowering temperature.
With the increase in temperature, the pressure required to liquefy a gas also increases. This temperature was the maximum temperature at which a gas appears in the form of a liquid. It is the critical temperature or TC. So, the necessary form of liquefaction is that the temperature of the gas must be below its critical temperature. When high pressure is applied to a gas, it gets compressed and when we lower its temperature it gets cooled.
Causes of liquefaction of gases –
Gases are hard to transport. Due to their physical properties, it is approximately impossible to move them from one place to another. For a similar reason, the gas is malformed into a liquid. The study of liquefaction of gases tells us about changes in the properties and structure of a gas. It also gives important information about the formation of substance in general.