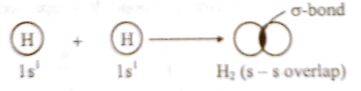A sigma bond is formed by end to end overlap of two s-orbitals, one s and one p-orbital or two p-orbitals. It is denoted by ‘σ’. The single electrons from each atom’s orbital combine to form an electron pair creating the sigma bond.
Fig Formation of H2 molecule by sigma (σ) bond