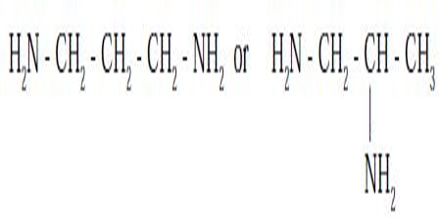Ligand isomerism arises from the presence of ligands which can adopt different isomeric forms. An example is provided by diamino propane, which may have the amine groups in the terminal (1,3-) positions or in the 1,2-positions.
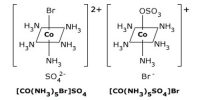
Ligand isomerism is a type of structural isomerism in coordination complexes that arises from the presence of ligands which can adopt different isomeric forms. Another example of such isomerism is shown by the ions, bis(1,3-diaminopropane)platinum(2+) and bis(1,2-diaminopropane)platinum(2+),
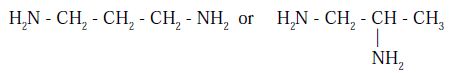
An example is provided by diaminopropane, which has two isomers that differ in the connectivity of the amine groups, 1,2-diaminopropane and 1,3-diaminopropane, so that two complexes that each feature a different isomer would be ligand isomers.