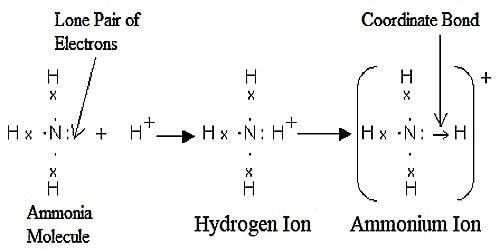
Formation of Ammonia ion (NH4)
In ammonia, there are three single N-H bonds and a lone pair on the nitrogen atom. When it reacts with H+ ion, the lone pair is used to form a coordinate bond between the nitrogen atom and hydrogen ion. Thus ammonium ion is formed.
The electrons of nitrogen have been denoted by a dot and those of hydrogen atoms by the cross. So by definition, there are three covalent N-H bonds and one N → H coordinate bond in NH4+ ion.
Once the ammonium ion has been shaped it is unattainable to tell any dissimilarity between the dative covalent and the ordinary covalent bonds. Although the electrons are shown differently in the diagram, there is no difference between them in certainty.
Covalent bond occurs between two non-metals and involves the sharing of electrons between two non-metal atoms
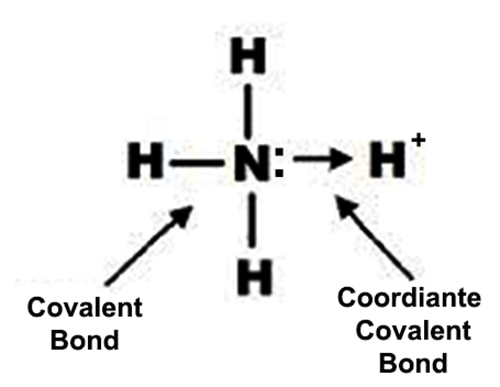
Bond in NH4+ ion
NH3 (Ammonia) is a covalent compound. In NH3 hydrogen can form 1 covalent bond while nitrogen can form 3 covalent bonds. When the ammonium ion, (NH4+), is produced, the fourth hydrogen is attached by a dative covalent bond, because only the hydrogen’s nucleus is transferred from the chlorine to the nitrogen. The hydrogen’s electron is left behind on the chlorine to form a negative chloride ion.
As nitrogen and hydrogen both are non-metallic compounds and does not have a tendency to donate their electron, so ‘Ammonia’ cannot be an ionic compound. Because ionic compounds have the propensity to provide their electron for the construction of ionic bond.