We are to use various materials in doing different work. We use of thousand of materials, starting with the water to wash our hands and face just after getting up from bed in the morning. We bring to use various type of food, crockery, clothes, toy, stone, cycle, football, marble, book etc. Of these, some are soft, some are hard, some are shiny, some are round and some are flat. But all of those are matter. All of these materials occupy space and have mass. So we can say that things which occupy space and have mass are called matter.
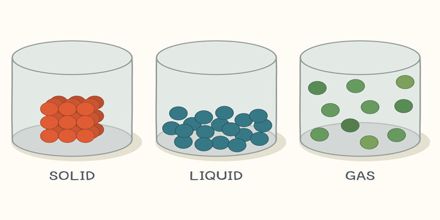
There are numerous types of matters in the world and they are classified in various ways. Among these, one of the classifications is based on the state of the matter. Let us take an example, when a piece of ice is kept in a pot, what happens? It is converted into water. Again we can convert the water into vapor by heating it up. So, it appears that water can be found in three different states. e.g.- ice, water and vapor. When water remains in the form of ice, it is in solid state. When it remains water, it is in liquid state. Again when it becomes vapor or steam, it takes gaseous state. So matter is divided in to three groups depending on its states. They are:
Solid; liquid and Vapor/gas
Now the question is what characteristics are responsible for making a matter solid, liquid or vapor?
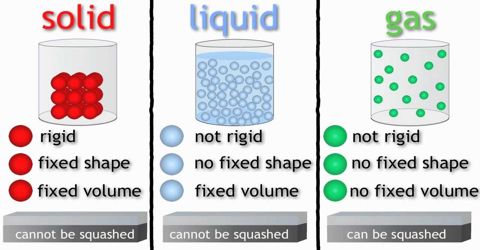
A solid body has a definite size. The space occupied by a body is its volume. As all solid bodies occupy space, they all have volume. The size and volume of a solid body can’t be changed easily. It is highly rigid, that is, it has rigidity. Although some of the solids have less rigidity (For example: mustard seed, boiled rice, and banana).
A liquid has no definite size. It holds the size of the container where it is kept. Liquid has a definite volume. Because it occupies space like solid. Its volume can be also measured. Does this volume changes? No, although depending on the size of the container the size of the liquid changes but the volume remains the same. As a liquid has no definite size, its size is changeable. Therefore, it can be said that the liquid is not rigid like solid e.g. liquid has no rigidity.
Let take the example of air to understand the property of gas. Like air no gas has any definite size. Is there any definite volume of a gas? Think of two cylinders, one small and another large. Now if you keep the same amount of gas in both the cylinders, the gas will occupy the space whole area of the small cylinder as well as of the large cylinder. Then it can be said that when the same amount of gas is kept in, a small cylinder, its volume is small and it is kept in the large cylinder, its volume is large. That means, the volume of the gas is the volume of the container in which it is kept. So gas has no definite size and volume.
In addition to that, some other characteristics which can be taken into account for classifying matters are density, rigidity, flexibility, thermal conductivity and electrical conductivity.