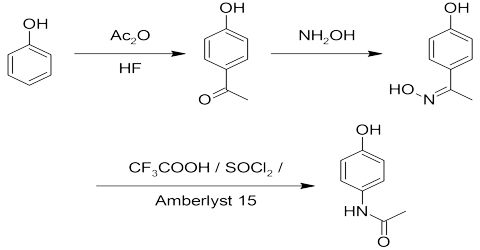
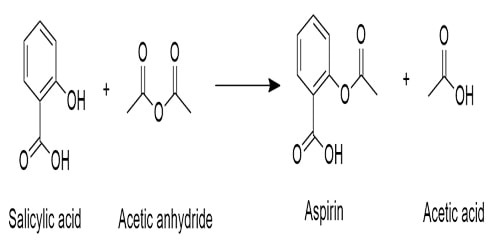
- Production of Aspirin
The acid anhydride is used as an acylation reagent. When phenol is heated with Carbon dioxide (CO2) and Sodium hydroxide (NaOH) at high temperature and high-pressure Salicylic acid are produced. Then salicylic acid is refluxed with ethanoic anhydride to produce aspirin. Aspirin is the ester of salicylic acid & ethanoic anhydride.
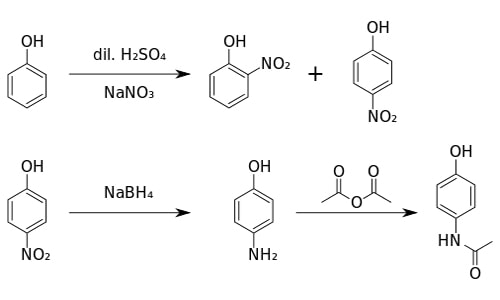
- Production of Paracetamol
4-aminophenol reacts with ethanoic anhydride to produce paracetamol (4-hydro acetinalide)
Aspirin is both an organic ester and an organic acid. It is used extensively in medicine as a painkiller (analgesic) and as a fever-reducing drug (antipyretic). When ingested, acetylsalicylic acid remains intact in the acidic stomach, but in the basic medium of the upper intestinal tract, it hydrolyzes forming the salicylate and acetate ions. However, its additional physiological effects and biochemical reactions are still not thoroughly understood.