In a modern Periodic Table the elements are written in order of increasing atomic number, not increasing R.A.M. This leads to Periodic law which states that “the properties of the elements are a periodic function of their atomic numbers”.
A group is a column, down the periodic table. In other words, vertical columns in the Table are called Groups. Elements in the same Group have similar properties. Elements in the same group generally have the same number of valence electrons in the highest energy level of their atoms. Groups are numbered from 1 to 18 and Groups 1, 2 and 13 to 18 are the Main Groups.
A period is a row, across the periodic table. In other words, horizontal rows in the Table are called Periods. Periods are numbered from 1 to 7. Each Period corresponds to a new value of n, the principal quantum number. The nature and properties of the elements change in a similar manner across each period.
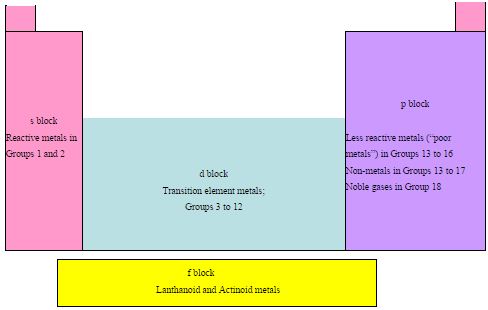
It is useful to split the Periodic Table into blocks of similar elements with similar structure and properties.