Measurement of Elevation of Boiling Point by Cottrell’s Method
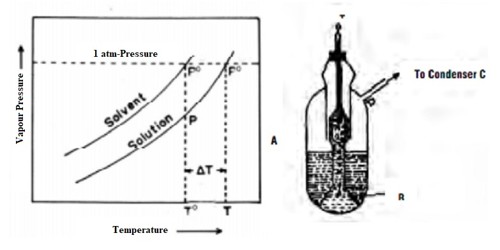
Cottrell’s method: Cottrell (1910) devised an ingenious method for measurement of boiling point in which superheating was completely eliminated the assembly is shown in Figure. A funnel with two or three-pronged tubes fits into the boiling tube in such way that the ends of the ‘U’-shaped pronged tubes lie a little above the thermometer bulb, as shown in the figure.
Figure: Cottrell’s Apparatus
The solvent-layer remains well below the thermometer bulb. On boiling, the liquid is forced through the pronged tubes and is pumped on the thermometer bulb and form a continuous layer of the boiling liquid on the bulb. This pumping device followed by the election of liquid on the tube eliminates superheating. The thermometer, therefore, records the true boiling point. Other experimental procedures air identical with the Beckmann method. This method gives a more reliable measurement of the boiling point. In all boiling point measurements corrections due to change in the barometric reading, if any, should be incorporated.
Further Explanation,
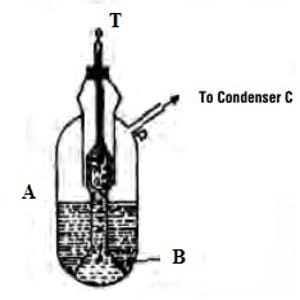
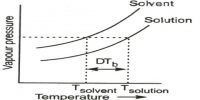
The apparatus (Fig.) consists of a boiling tube (a) which is graduated and contains a weighed quantity of the fluid under assessment. The boiling point of fluid might be defined as the temperature at which its vapor pressure becomes equivalent to atmospheric pressure, i.e., 760 mm. A reversed funnel tube (b) placed in the boiling tube collects the bubbles rising from a few fragments of a permeable pot placed within the liquid. Since the accumulation of a non-volatile solute lowers the vapor pressure of the solvent, solution at all times has inferior vapor pressure than the solvent and therefore it must be heated to a higher temperature to make its vapor pressure equivalent to atmospheric pressure with the consequence the solution boils at a superior temperature than the pure solvent.
When the fluid starts boiling, it pumps a stream of a liquid and vapor over the bulb of the Beckmann thermometer (f) held a small above the liquid exterior. In this method, the bulb is covered with a thin layer of boiling liquid which is in balance with the vapor. This ensures that the temperature interpretation is accurately that of the boiling liquid and that superheating is minimum. After formative the boiling point of the pure solvent, a weighed amount of the solute is added and the process is frequent for another interpretation. The vapor of the boiling liquid is frozen in a condenser (C) which has a transmission of water through (d) and (e). The cooled liquid drops into the liquid in (a).