Lyophobic and Lyophilic Sols
A rough classification of sols, based on their methods of preparation, is sometimes made, e.g., association or condensation colloid, dispersion colloid and molecular colloid. But it is not a suitable classification because disintegration or dispersion colloid in its properties is not different from association colloid. The final products are the same, only the method of preparation is different. However, molecular colloids have some distinguishing properties. So, only two types of colloids are generally recognized.
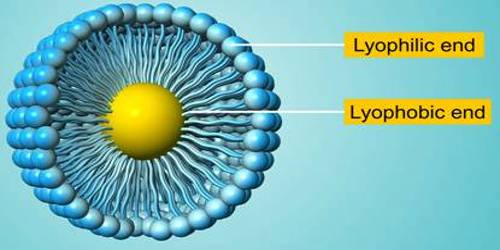
- Lyophilic colloids are liquid loving colloids (Lyo means solvent and philic means loving). Example: Sols of organic substances like gelatin, gum, starch, and proteins.
- Lyophobic colloids are liquid hating colloids (Lyo means solvent and phobic means hating). Example: Sols of inorganic substances like Arsenic (As2S3), Iron (Fe(OH)3) and Platinum.
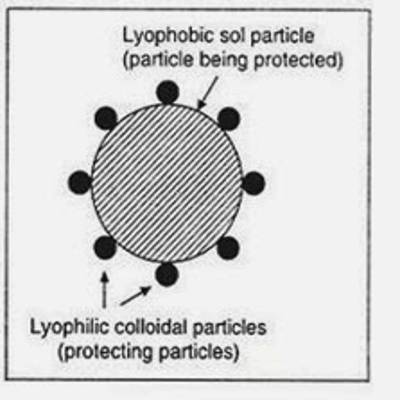
The association or dispersion colloids are considered to be lyophobic or solvent hating whereas the molecular colloids are lyophilic or solvent loving. The colloidal electrolytes, although in many cases are similar in their properties to lyophilic colloids, constitute a separate class. We have thus three types –
- Lyophobic
- Lyophilic and
- Colloidal electrolytes.