Important Concepts in Thermodynamics
The laws of thermodynamics and many quantitative relationships deduced therefrom are based on some fundamental concepts. It is imperative to understand these concepts before discussing the principles of thermodynamics.
System, boundary and surroundings
The portion of the physical universe which is under thermodynamic consideration is called a system. It usually consists of some form of matter undergoing a change. The system is confined to a definite place in space by the boundary which separates it from the rest of the universe, the surrounding. The boundary is actually the interface between the system and the surrounding. The boundary may be real or imaginary. For example, let consider a beaker containing water. In this case the beaker with the water is the system. The rest of the universe outside the beaker is the surrounding. The wall of the beaker is the boundary. However, in practice the immediate vicinity of the system is considered as the surrounding.
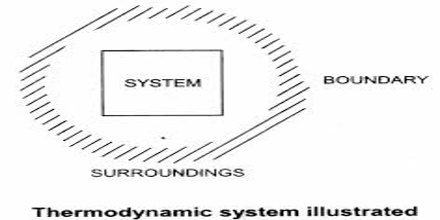
There are three types of systems depending on the nature of the boundary open, closed and isolated.
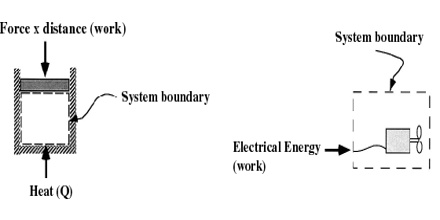
Open System: The system is called open when both energy and mass can pass between the system and the surroundings across the boundary.
Closed System: If mass cannot pass between the system and the surroundings across the boundary, but energy can be exchanged with the surroundings, it is called a closed system. Energy may pass through the boundary either as heat or as some form of work. A sealed beaker containing water is an example of closed system.
Isolated System: A system is said to be isolated when the boundary prevents the exchange of both energy and mass with the surroundings. A sealed flask thermally mechanically and electrically isolated from the surroundings is an example of closed system. The boundary in this case must be thermally and electrically insulated.
A system can be either homogeneous or heterogeneous.
If the composition of the system is uniform throughout, it is called homogeneous. Pure liquid, pure gas, pure solid, mixture of gases or a true solution – are few examples of homogeneous systems. If the composition of the system is not uniform throughout and consists of two or more phases, it is called a heterogeneous system. Mixture in insoluble liquids, mixture of solids, mixture of liquid and its vapor are few examples of heterogeneous systems. There are phase boundaries between different phases or components in heterogeneous systems.