Hydrogen bonding
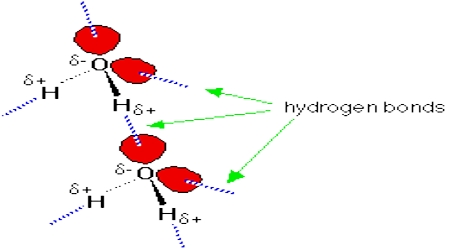
Strong forces of attraction exist between molecules containing a hydrogen atom bonded to a highly electronegative element such as nitrogen, oxygen or fluorine. This can be deduced by comparing the boiling point of water (H2O) with that of hydrogen sulphide (H2S). Water boils at 100°C while at room temperature hydrogen sulphide is a gas. The attractive forces between H2O molecules must be much stronger than that between hydrogen sulphide molecules. Water is a highly polar molecule and the strong attraction between water molecules is attributed to dipole-dipole interaction. Such attractive forces are named hydrogen bonding because of the strength of such attractions as compared to other dipole-dipole attractions.
Hydrogen bond is defined as follows: In compounds where a hydrogen atom is covalently bonded to a highly electronegative atom such as nitrogen, oxygen or fluorine the strong attractive force between a hydrogen atom of one molecule for the electronegative atom of another molecule is called the hydrogen bond.
The large electro negativity difference between the hydrogen atom and the electronegative atom gives rise to a partial positive charge on the hydrogen atom and a partial negative charge on the other atom joined by a covalent bond. As a result a stabilizing interaction between two or more molecules is developed that binds the molecules together. A common example of H-bonding is found in water:
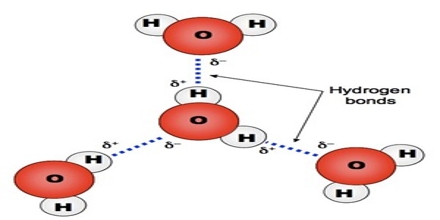
It should be understood that hydrogen bond is an intermolecular force and not a bond as is understood in the cases of ionic or covalent bond. No transfer or sharing of electrons occurs.
Evidences of hydrogen bonding are found throughout nature. Hydrogen bonding explains why the density of ice is less than that of liquid water. In the liquid state the water molecules joined by hydrogen bonds constantly change partners. When water starts freezing the hydrogen bonds between the molecules get fixed and in the solid state, as the molecules cannot move, the hydrogen bonds between molecules get fixed in position. In the solid state (ice) each oxygen atom is surrounded tetrahedral by four hydrogen atoms: two forming covalent bonds with the O atom and are close so it to form H2O molecule and two from other H2O molecules farther away from it forming two hydrogen bonds. The result is a three-dimensional structure with empty space. This is why Ice is less dense than water. When ice melts and liquid is formed again by Hydrogen bonds we constantly breaking and forming so that molecules can get close to each other giving rise to the liquid. This is a unique property of water and is very important to life on earth.