Titration is the process for the determination of the unknown concentration or a solution volumetrically. In this process a solution of the primary standard substance of a particular volume is taken into a cortical flask using pipette Then 1-2 drops of the indicator is added to it. The solution of unknown concentration is taken into burette and added this solution to the conical flask for reaction. After completing the reaction the colour of solution become changed.
Then using the known volume of primary and secondary standard solution and the known concentration of the primary standard solution, the unknown concentration is determined.
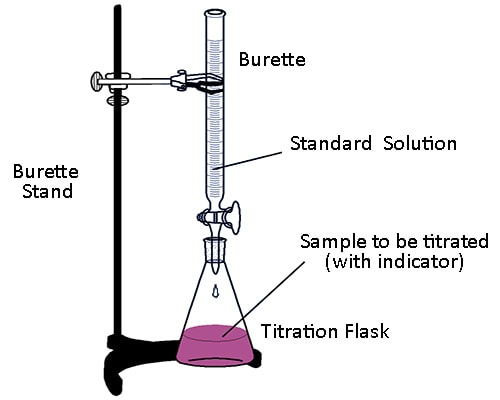
Fig: Titration