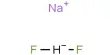
Le – Chateliar principle: When a system is at equilibrium, a change in any one of the factors (such as temperature, pressure, concentration) upon which the equilibrium depends will cause the equilibrium to shift in a direction such that the effect of the change is diminished.
Statement of Le Chatelier’s Principle: “If a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to counteract the change.”
Suppose you have an equilibrium established between four substances A, B, C and D.
A + 2B ↔ C + D
Main Three Principles:
- changing the concentration of one of the components of the reaction
- changing the pressure on the system
- changing the temperature at which the reaction is run.