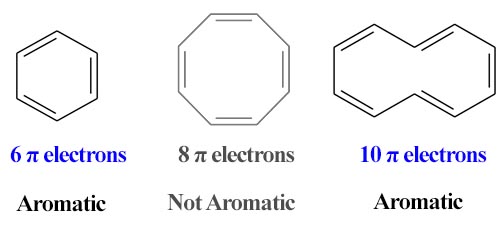
Huckel Rule: The cyclic π molecular orbital (electron cloud) formed by overlapping of p orbitals must contain (4n+2) electrons, where n, integer = 0, 1, 2, 3 etc. This is known as Huckel rule. In 1931, Erich Huckel hypothesized that, monocyclic planar compounds that contained carbon atoms with unhybridized atomic p orbitals would possess a closed bond shell of delocalized π electrons.
According to this rule,
- An aromatic compound must be cyclic and planner.
- Each atom in an aromatic ring has a-p orbital. These p-orbitals must be parallel so that a continuous overlapping is possible around the ring.