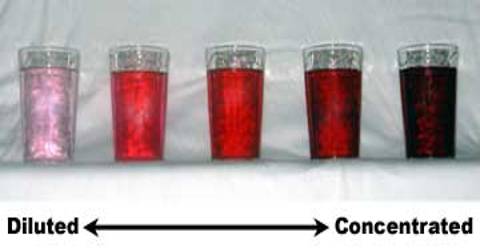
A liquid with a high solute concentration is called a concentrated solution. The dissolved salt from a well in the drinking water is a dilute solution. It is not possible to say whether a colorless watery solution is dilute or concentrated by taking a look at it. But in case of colored solutions of different concentration level, it is possible to say which one is dilute and which one is concentrated by looking at them, just like the way we can differentiate concentration and dilute dal (note that, dal is not a solution but a heterogeneous solution). Now we will see how we can differentiate between concentrated and diluted colored solutions.
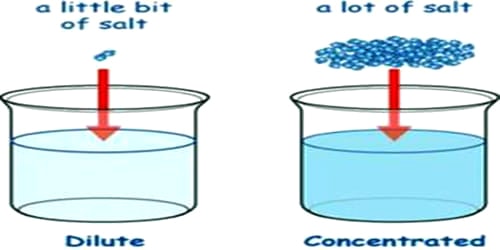
Dilute solution: When a small amount of solute is dissolved in a relatively large quantity of solvent, then the solution is called a dilute solution.
Concentrated solution: When the amount of solute dissolved is relatively large as compared to the quantity of solvent, then the solution is called a concentrated solution.
Task: To make colored watery solutions of different concentrations and to differentiate between concentrated and dilute solutions. The main difference between the dilute solution and concentrated solution is that the dilute solution contains less solute and the concentrated solution contains more solute.
Required accessories: Three test tubes, test tube holder, measuring flask, spoon, copper sulphate, and water.
Procedure: Take three clean test tubes. Now place the test tubes in a test tube holder one after another. Take 5 milliliters of water in each tube using a measuring flask. Now add in the first test tube 1 spoon, in the second test tube 2 spoon, and in the other 3 spoons of copper sulphate. Then shake the test tubes well till the grains of copper sulphate is completely dissolved. Again set the test tubes in the test tube holder at their own places before.
Analysis: The terms dilute solution and concentrated solutions are vague as they lack quantitative preciseness. Do all the solutions look the same blue? No, they do not. The solution looks less blue in which you added less copper sulphate. That means it is a less dilute solution. In this way, as you increase the amount of copper sulphate, the color of the solutions gradually becomes deeper. That Main, the solution has become more concentrated form diluted with the increase in the amount of copper sulphate. You can now differentiate between dilute and concentrated solutions by preparing a solution of potassium permanganate and potassium dichromate in the same method.
Explanation: Dilute acids will be, for example, 80 or 90% water, with only 10 – 20% of the weight being the acid itself. Concentrated acids have much lower water contents – concentrated sulphuric acid is typically 4% water, 96% acid. Concentrated hydrochloric acid is 37% HCl (HCl itself is a gas, so 37% w/w is about the maximum you can dissolve in water).