Effective Separation by Chromatography
An effective separation by chromatography requires a judicious choice of the adsorbing material and the solvent, and they are to be selected on the basis of the properties of the solutes. Excellent tables of solvents and column materials to meet different requirements are available.
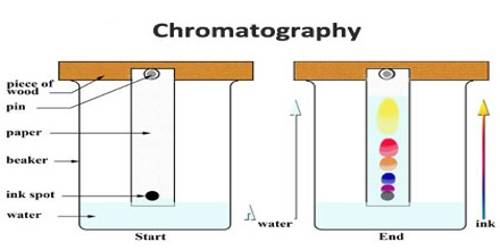
In addition to using the column for chromatographic separation simple filter paper strips can be used with good results, and in some cases with spectacular success. This is known as paper chromatography. In this method, a small dot, usually of the size of a millimetre, is made with the solution containing different solutes near one end of a strip of chromatographic filter paper. The solvent is then allowed to flow along the paper strip slowly up to a definite length of the paper. The different solutes move with different speeds and a separation is affected; if the solutes are colorless they can be rendered visible by spraying the paper with suitable reagents that form colored compounds. Alternatively, in many cases, the solutes can be rendered luminescent and thus visible by irradiation with ultraviolet rays in a dark room. The luminescence is due to fluorescence. Ordinary blue-black fountain pen ink is a good sample for paper chromatography. Most of them contain three or four colored materials and they become easily recognizable in a paper chromatogram. If the dot is formed near the
upper edge and the solvent is allowed to flow downwards, the method is called descending chromatography because the constituents descend; if the dot is formed near the lower end of the paper and the solvent moves upward by capillary action, the method is called ascending chromatography. In paper chromatography the adsorbing material is cellulose in all cases and, therefore, is of somewhat limited application, whereas in column chromatography there are some inherent difficulties. The limitations of both the methods are eliminated in thin layer chromatography. In this method, a thin layer of a suitable adsorbing material is made on a rigid support and normal chromatographic techniques employed.
The principle of different adsorption is nowadays extensively used in gas phase or vapor phase chromatography. The elution is done by and then earner gas, almost universally helium.