Determination of Osmotic Pressure by Modern methods
Abbe Nollet (1748) was the first to observe the phenomenon of osmosis and made measurements of osmotic pressure. His measurements with pig’s bladder as the semi-permeable membrane gave only semi-quantitative results as the pig’s bladder is not a good semi-permeable membrane.
Modern methods – Different types of membrane osmometers have been developed for quick and accurate measurements of osmotic pressure. The membrane osmometers generally used for the determination of osmotic pressures may be divided into two classes:
(i) Static osmometer: This type of osmometer is based on the attainment of equilibrium osmotic pressure due to diffusion of solvent through a membrane.
(ii) Dynamic osmometer: In this case the flow of solvent through the membrane prevented by the application of appropriate pressure to the solution side.
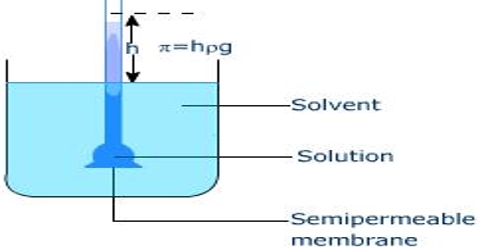
A simple form of static osmorneter is illustrated in Figure. The solution under investigation is taken in the inner bulb which carries at one end the semi permeable membrane like cellophane. The other end of the bulb is attached to a graduated capillary tube. This bulb is placed inside a wider vessel which contains the selves. The solvent passes into the solution and causes the liquid to rise until an equilibrium height, h, is attained. After incorporating necessary corrections the osmotic pressure is calculated.
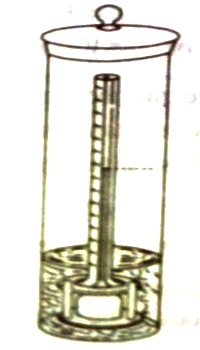
Figure: A modern osmometer
Dynamic osmorneter, like vapour phase osmometer is particularly used for the determination of molar mass of high molecular mass polymers of macromolecules.