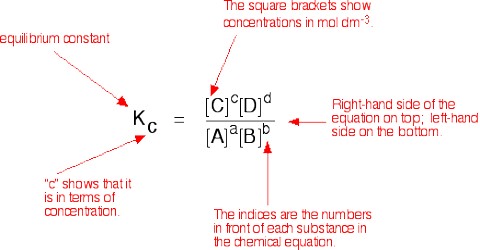
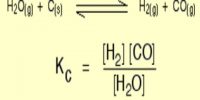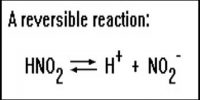Determination of Equilibrium Constants
In determining the equilibrium constants the concentrations of the various species at equilibrium are to be known. This has to be done in a manner so that the equilibrium is not disturbed by the method of analysis used for the purpose. Analytical procedure should be so chosen that the concentrations of all the species may be obtained from the initial concentrations and from the experimental determination of a minimum number of chemical entities at equilibrium. Often this calls for a considerable amount of ingenuity on the part of the chemist.
Many chemical and physical methods have been used for the determination of the equilibrium constants of reactions. Physical methods based on measurement of absorption of light, refractive index, pressure or volume change, electrical conductance etc. are more suitable for the purpose. In chemical methods often the equilibrium is frozen (quenched) and then the equilibrium mixture is analyzed. The freezing of the equilibrium is achieved by sudden cooling of addition of an excess of a chemical reagent or simply by large dilution. In any case, the analysis has to be performed very rapidly. In some cases a suitable precipitating agent may be added to remove the reactants or the products and then the precipitate is quickly filtered out under a suction pump.