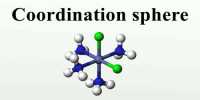
Uses of coordination compounds
(a) Dyes and Pigments
Coordination compounds have been used from the earliest times as dyes and pigments, for example madder dye which is red, was used by the ancient Greeks and others. It is a complex of hydroxyanthraquinone. A more modern example is the pigment copper phthalocyanine, which is blue.
(b) Analytical Chemistry
The coordination compounds used for various purpose, in the laboratary are as follows.
- Colour Tests: Since many complexes are highly coloured they can be used as colourimetric reagents e.g. formation of red 2,2’-bipyridyl and 1,10-phenanthroline complexes as a test for Fe2+
- Gravimetric Analysis: Here chelating ligands are often used to form insoluble complexes e.g. Ni(DMG)2 and Al(oxine)3.
- Complexometric Titrations and Masking Agents: An example of this is the use of EDTA in the volumetric determination of a wide variety of metal ions in solution, e.g. Zn2+, Pb2+, Ca2+, Co2+, Ni2+,Cu2+, etc. By careful adjustment of the pH and using suitable indicators, mixtures of metals can be analysed, e.g. Bi3+ in the presence of Pb2+. Alternatively, EDTA may be used as a masking agent to remove a metal ion which would interfere with the analysis of a second metal ion present.
(c) Extraction of Metals
Sometimes certain metals can be leached from their ores by formation of stable complexes e.g. Ag and Au as complexes of cyanide ion.
(d) Bio-Inorganic Chemistry
Naturally occurring complexes include haemoglobin, chlorophyll, vitamin B12 etc.
Therapeutic chelating agents are used as antidotes for heavy metal poisoning. EDTA and other complexing agents have been used to speed the elimination of harmful radioactive and other toxic elements from the body. (e.g. Pb2+). In these cases a soluble metal chelate is formed.
(e) Chemotherapy
An example here is the use of cis-Pt(NH3)2Cl2 as an anti-tumour drug.
(f) Synthetic detergents
Synthetic detergents containing chelating agents such as tripolyphosphate. The chelating agent sequesters hard-water cations, rendering them incapable of interfering with the surfactant.