Comparison of metallic conduction and electrolytic conduction
Conductors of electricity may be divided into two groups according to the mechanism by which electricity is carried through them. In a metallic conductor, the charge carriers are electrons and, under the influence of an external electric field, they acquire some average drift velocity in the direction opposite the field.
Metallic conduction
- The current is solely carried by the electrons in the conduction band.
- The velocity of the electrons is very large.
- During passage of current no chemical reaction occurs; only heating effect is produced.
- Specific conductance of many metals are quite high, they are very good conductors of electricity.
- Temperature co-efficient in general is negative (alloys show complex behavior).
- Ohm’s law applies.
- Conductance may be measured by d-c or a-c current.
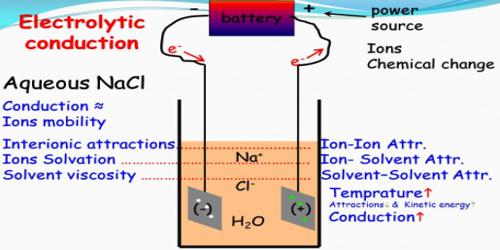
Electrolytic conduction
- The current is carried by both cations and anions.
- Ionic velocities are much smaller than electron velocities.
- Passage of current brings about chemical reactions; heat is also evolved.
- Specific conductance is low; they are moderately good conductors.
- Temperature co-efficient is positive.
- Ohm’s law applies
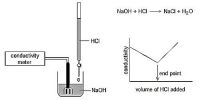
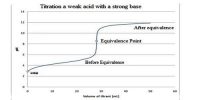
- Conductance is measured by a-c source; d-c current can be used only by elaborate arrangements.