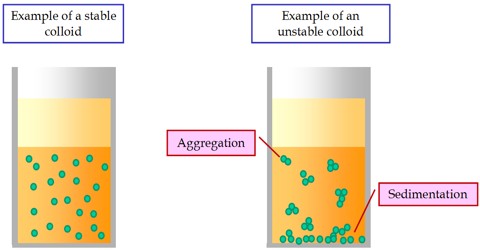
A colloid is solutions that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution. In case of suspensions we saw that the small particles of a components remains in floating condition but if it is kept for a long time then the particles gathers as sediments. However, cannot it he the case that tilt particles are so small and light that they will never be able to gather at the bottom of the pot and will always remain in floating or suspending condition? Yes, surely it can be. Hence that type of mixture where very small particles of a substance remains in floating or suspended condition in the middle of the particles of another substance and even if it is kept at rest it will never gather as sediment that is called colloid.
The existing components of a colloid are not dissolved in to one another but remain scattered. In colloid, the main component or the component which is more in amount is called continuous phase and which remains less in amount is called dispersed phase. Milk is a colloid, which is made of water and fat. The small particles of fat (dispersed phase) remains scattered in water (continuous phase), which is not seen by naked rye but with the help of a microscope. The picture of milk found with the help of a microscope is shown in the figure.
Fog is another colloid like milk, where small water particles remain scattered in air.
Again our quite known aerosol is one kind of colloid, where liquid particles of insect killers float in the air. Now the question is, to make a colloid; how much small should the floating small particles be. Usually, the size of the existing floating particle is 1-1000 nanometers. (1nm = 10-9m). m=10-6m).