In 1897, British physicist J.J. Thomson measured the ratio of electrical charge (e) to the mass of electron (me) by using cathode ray tube and applying electrical and magnetic field perpendicular to each other as well as to the path of electrons (Figure). Thomson argued that the amount of deviation of the particles from their path in the presence of electrical or magnetic field depends upon:
(i) the magnitude of the negative charge on the particle, greater the magnitude of the charge on the particle, greater is the interaction with the electric or magnetic field and thus greater is the deflection.
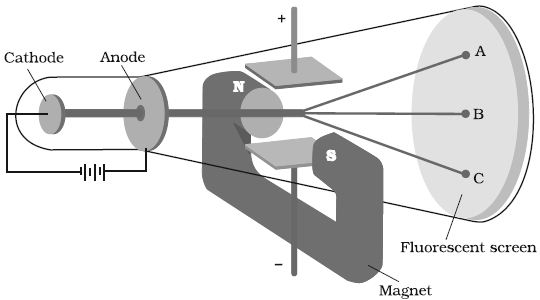
(ii) the mass of the particle — lighter the particle, greater the deflection.
(iii) the strength of the electrical or magnetic field – the deflection of electrons from its original path increases with the increase in the voltage across the electrodes, or the strength of the magnetic field.











