Characteristics of unsaturated vapour pressure
An unsaturated solution is when the solute can’t dissolve in the solvent. For example, if you have a glass of water, and you pour something like vegetable oil into it, it will not dissolve. So that makes the solution unsaturated. The maximum limit of water vapour that a given quantity of air can hold at a particular temperature is termed as a saturated vapour. In such a case, relative humidity will be 100 percent. For all other cases, where the maximum limit of vapour is not reached, the vapour thus formed is termed as unsaturated vapour. When the air does not contain the maximum amount of water vapour it is said to be unsaturated for that temperature. Unsaturated-saturated liquid and vapour flow modeling show that the topsoil may act as a capillary barrier to infiltration, thereby promoting desiccation of the underlying compacted till.
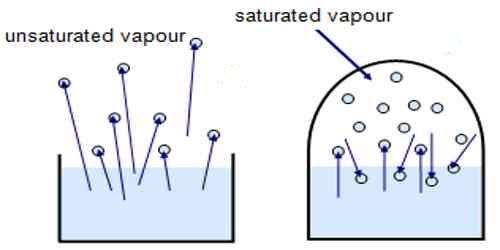
- Vapour in a dosed space is said to be unsaturated at a given temperature if the pressure is less than the saturated vapour pressure at that temperature.
- It can be created in a closed or open space. The process comprises providing a pressure within the chamber which is less than atmospheric and providing an unsaturated treatment vapour within the chamber.
- If there exists some vapour in a confuted space but there is no liquid in that space, then that vapour can either be unsaturated or just saturated. If some vapour becomes liquid due to the decrease of the volume of that space, then that vapour is just saturated, otherwise unsaturated.
- Unsaturated vapour follows Boyle’s law and Charles’s law.
- By decreasing temperature, a fixed amount of unsaturated vapour can be made saturated.
- Unsaturated vapour does not remain in equilibrium with its liquid. A liquid that can be condensed into fine siliceous capillaries from unsaturated water vapour has properties very different from those of ordinary water.