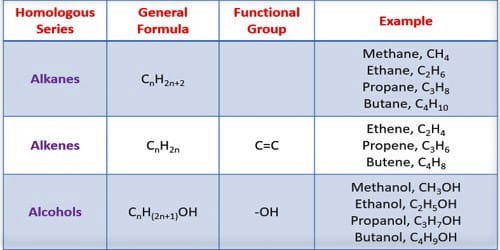
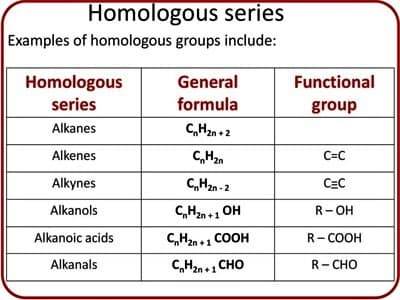
A homologous series in organic chemistry is a group of organic compounds [compounds that contain carbon (C) atoms] that diverge from each other by one methylene (CH2) group. For example, methane, ethane, and propane are part of a homologous series.
Characteristics of a homologous series
The compounds of the homologous series have some characteristics – they are as follows:
- General formula:
All the members of a series can be represented by the same general formula. This gives the homologous series name. All the members of a homologous series have a similar structural formula. The molecular formula of dissimilar members of a homologous series differs from preceding and subsequently member by [CH2]. For example, Alkane: CnH2n+1; Alkene: CnH2n; Alkyne: CnH2n-2; Alcohol: CnH2n+1OH; Ether: CnH2nO. [Where n = number of carbon atoms].
- Same functional group:
It has a similar functional group. All members of a series contain the same functional group i. e. alcohol has -OH group and amine has -NH2 group etc. They all contain the same functional group.
- The difference in molecular mass:
The difference in molecular mass of two consecutive members of the series is 14 unit i.e. CH2. The consecutive members differ in their molecular formula by [CH2] group; they differ in molecular weight by 14 units.
- Preparative method:
It can be prepared by similar chemical methods. All the members of the series can be prepared by common methods – only in special cases, different methods may be used. So, generally, all the members of a series can be prepared by using the same method.
- Identical chemical properties:
Similar types of chemical properties are observed due to the same functional group. They show similar chemical properties. However, with increasing molar mass the chemical reactivity decreases to some extent. They display similar chemical properties. All the members of a homologous series have similar chemical properties due to similar functional groups.
- Regular order in physical property:
With an increase in molar mass, there is a gradual change in physical properties. They demonstrate a stable transform in physical properties from one member to the next. They show graduation in physical properties such as melting point, boiling point, density or solubility. The physical properties of the members of a homologous series differ with the increase in atomic weight. For example, boiling and melting temperatures increase with molar mass-increment but solubility decreases.