The Bronsted-Lowry theory discusses acid-base reactions in terms of proton-transfer only. Note that an H atom only has one proton and one electron, as such a proton is exactly the same as an H+ ion. The Bronsted-Lowry theory states that an acid is defined as a proton donor and a base is defined as a proton acceptor.
Conjugate acid-base pairs: The Bronsted-Lowry theory identifies acids and bases as conjugate pairs, which transform into each other with the loss or gain of a proton. It views all acid-base reactions as equilibrium as follows:
HA → H+ + A–
The general form of an acid can be written HA. When an acid donates a proton, it becomes the conjugate base A-. In the reverse reaction, when a base A– accepts a proton, it becomes its conjugate acid HA.
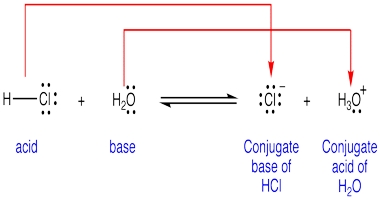
Summary of Bronsted-Lowry theory
- An acid is a species which donates protons.
- A base is a species that accepts protons; OH- is only one example of a base.
- Acids and bases can be molecular substances or ions.
- Acid-base reactions are not restricted to aqueous solution.
- Some species can act as either acids or bases depending on what the other reactant is. This is known as being amphoteric.