Autocatalysis:
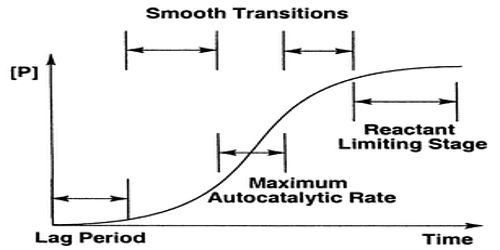
A single chemical reaction is said to have undergone autocatalysis, or be autocatalytic, if one of the reaction products is also a reactant and therefore a catalyst in the same or a coupled reaction. The reaction is called an autocatalytic reaction. It occurs often and since the surface gets covered the reaction is modified. The decomposition of SbH3 is a slow reaction on glass but the reaction is greatly accelerated by Sb itself. Consequently, the Sb formed as a result of the reaction accelerates the rate since Sb remains adsorbed on the glass surface. This is a case where the product of the reaction itself acts as a catalyst. Such reactions are called autocatalytic reactions and the phenomenon is known as autocatalysis.
The reverse phenomenon is, however, more common. The product of the reaction often covers a considerable portion of the surface and thereby retards the rate of the reaction. When ammonia is thermally decomposed on platinum surface and the hydrogen formed as a result of the reaction, 2NH3 → N2 + 3H2, is strongly adsorbed and covers the free platinum surface the reaction is slowed down.
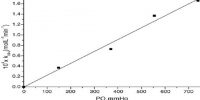
SO3, formed in the contact process, exerts a similar retardation effect where platinum is the catalyst. The rate is found to be inversely proportional to 0.5 power of the SO3 concentration. The surface is said to be poisoned and the phenomenon is known as auto-retardation. External gases, small solid particles carried over by a reacting gas etc., quite often poison the catalysts in many reactions and extreme care is needed to free the reaction gas from such poisons. Water vapor acts as a poison in NH3 synthesis by Haber’s process. Dust particles, arsenic compounds are strong poisons for platinum in the contact process for H2SO4 manufacture. NO acts as strong inhibitor for many reactions in gas phase where free radical mechanism is involved.
The kinetics of autocatalytic and auto-retarded reactions is quite complicated and often insolvable. In a number of reactions the concentration of the poison or the catalytic enters into the rate expression.