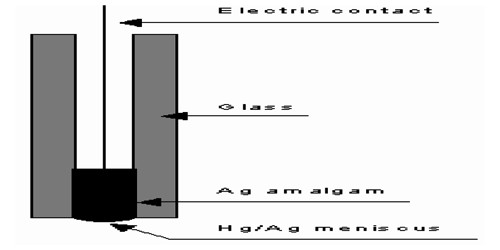
Amalgam electrode in Half-Cells
A half cell is one of the two electrodes in a galvanic cell or simple battery. For example, in the Zn-Cu battery, the two half cells make an oxidizing-reducing couple.
In many cases, it is convenient to form the metal electrode by using an amalgam, i.e., a solution of the metal in mercury. Sometimes when the metal is highly reactive, it is more convenient to use the metal in the form of amalgams. The activity of the metal is lowered by dilution with mercury. These electrodes are generally set up by placing the metal-amalgam in contact with a solution of metal ion. An example is the Cd-amalgam electrode used in the Weston standard cell. As shown in Figure, electrical contact is made by a platinum wire immersed in the amalgam.

The reaction is the same as in the metal-metal ion electrode. An amalgam electrode is of importance in those cases where the metal is too reactive to be used in the pure form. For example, one cannot make a sodium electrode with the pure metal as sodium reacts with water, but a sodium amalgam electrode may be set up easily. Unless the amalgam it saturated with respect to the solute metal the concentration of solute metal in the amalgam as well as the concentration of the metal ion must be given as the e.m.f. of the electrode depends on both. A sodium amalgam half-cell is represented as-
Na – Hg (C1│Na+ (C2)