Adsorption theory: This theory can be applied to heterogeneous catalysis only. As the name suggests the reactant is adsorbed on the surface of the catalyst and the effective concentration of the reactant is generally increased on the surface, the reactants are brought closer and the reaction speed is increased. However, it is now fairly well known that simple physical adsorption on the surface of the solid has practically nothing to do with catalysis. It is chemisorptions that is mainly responsible for catalytic activity. As mentioned the unused bonding capabilities of the surface atoms or ions may be utilized to bond molecules from the gas or solution phase to the surface of the solid. In practice, not all the atoms or ions on the surface are reactive and can adsorb gases or ions. The places where reacting molecules can get adsorbed are called active sites.
As per the transitional compound theory of catalysis, the reaction that is preferred is achieved with the structure of an intermediate compound and the subsequent decomposition of the similar into the preferred products. In this procedure, the catalyst is regenerated after the reaction is over.
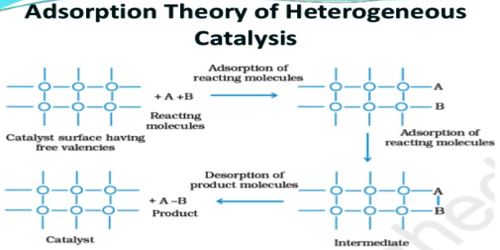
The extent of chemisorptions will, therefore, depend on the number of available active sites and on the nature of the reacting molecules. The number of active sites per unit of catalyst depends on the nature of the catalyst, on its method of preparation, and on its treatment before use.
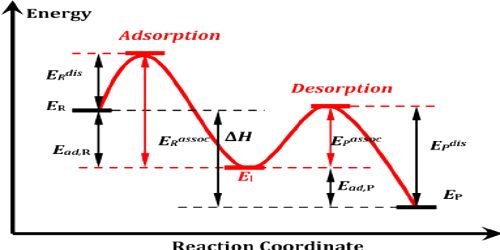
It is believed that the mechanism of heterogeneous catalysis on the surface of solid catalysts consists of five steps as follows:
- Diffusion of Reactant(s) to the Surface:
Reactant molecules diffuse to the solid catalyst surface. The reactants diffuse to the surface of the catalysts. The rate at which reactants will disseminate to the surface will be influenced by their bulk absorption and by the thickness of the border layer.
- Adsorption of reactants:
Reactant molecules are first physically adsorbed on the surface. They then become chemisorbed. Bonds are formed as the reactant(s) are adsorbed onto the surface of the catalyst. These molecules then get adhered to the suitable sites available for adsorption. The capability for an atom or molecule to stick to the surface is known, radiantly, as the Sticking Co-efficient.
- Chemical Reaction:
The chemisorbed molecules adjacent to each other react to produce the products. The reactants, when bound to the surface have a higher probability of reacting with each other, and after the reaction, they form an intermediate compound. Bonds form between the atoms and molecules on the exterior.
- Desorption of products:
This is the reverse of adsorption. After the reaction, the product molecules are at first chemisorbed on the surface. After this procedure, the transitional compound gets desorbed from the surface, which over again becomes obtainable for adsorption for other molecules to come. Bonds are busted as the product(s) desorbs from the surface. They then become physically adsorbed and finally break free from the surface.
- Diffusion of Product(s) away from the Surface:
The product molecules diffuse away from the surface. The transitional compound then disintegrates to form the last products, which then disseminate out of the interior pores and the outside surface of the catalyst. The products are then desorbed from the surface of the catalyst.