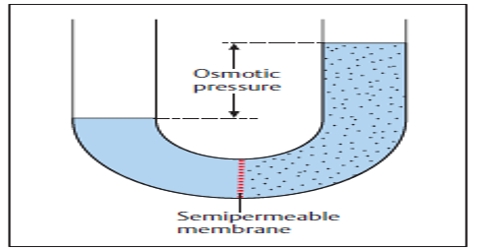
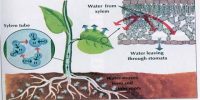
Osmotic pressure: Osmotic pressure can be defined as the pressure required to stop completely the movement of the solvent through the semi-permeable membrane. Osmotic pressure is the pressure created by water moving across a membrane due to osmosis. The more water moving across the membrane, the higher the osmotic pressure.
The more is the dissimilarity in the concentration of the solution, the more is the osmotic pressure. They gain water by osmosis through their roots. Water moves into plant cells by osmosis, making them turgid or stiff so that they are able to hold the plant upright.
Osmotic pressure is only permanent if the membrane is truly semipermeable, i.e. if it stops all solute molecules and only passes the solvent molecules.
Osmotic pressure is of essential substance in biology as the cell’s membrane is selective toward many of the solutes found in living organisms. When a cell is placed in a hypertonic solution, water essentially flows out of the cell into the surrounding solution thereby causing the cells to shrink and lose its turgidity.