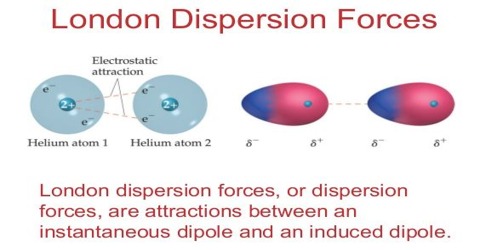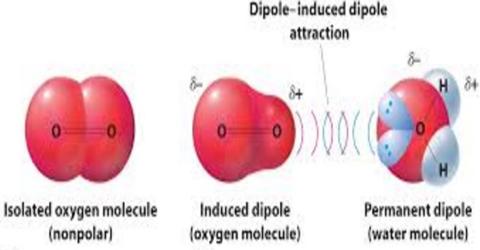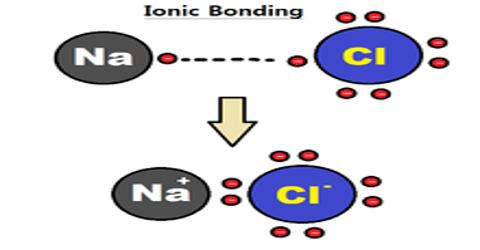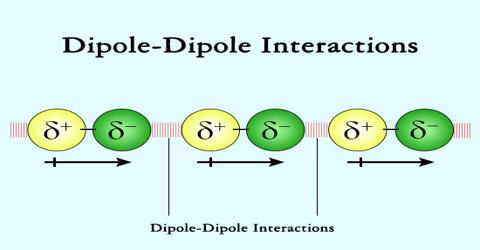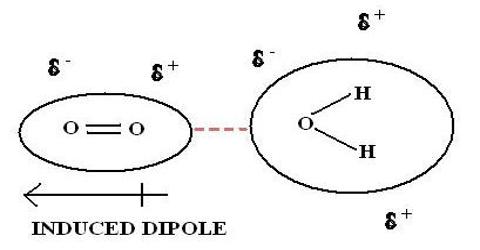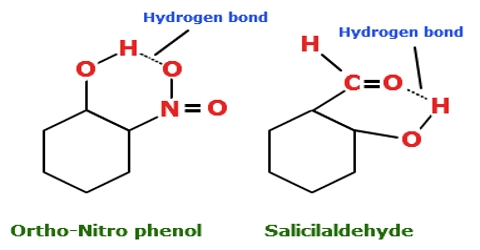
Chemistry
Intramolecular Hydrogen Bonding: Definition in terms of Inter-molecular Forces
Intramolecular hydrogen bonding (i.e. hydrogen bonding between the hydrogen atom in one part of the molecule with an electronegative atom like oxygen or nitrogen in…