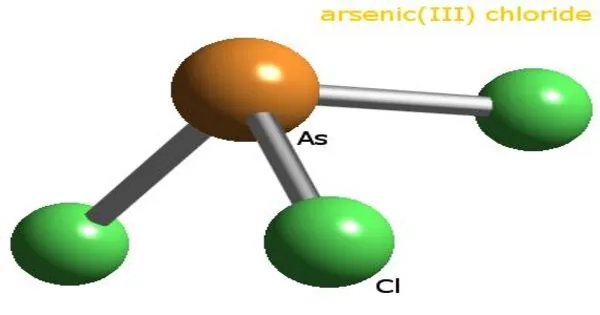
Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. It is a colorless to yellow liquid with a pungent odor. It is highly toxic and should be handled with extreme caution. This poisonous oil is colorless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.
Properties
- Chemical formula: AsCl3
- Molar mass: 181.28 g/mol
- Appearance: colourless oily liquid
- Density: 2.163 g/cm3, liquid
- Melting point: −13 °C (9 °F; 260 K)
- Boiling point: 181 °C (358 °F; 454 K)
- Solubility in water: Hydrolyzes
- Solubility: soluble in alcohol, ether, HCl, HBr, chloroform, CCl4
- Magnetic susceptibility (χ): -79.9·10−6 cm3/mol
- Refractive index (nD): 1.6006
Application
It is primarily used in organic synthesis as a reagent for the introduction of the arsenic group into other compounds. It can also be used in the manufacture of certain pesticides, pharmaceuticals, and dyes. However, its use is limited due to its toxicity and the availability of safer alternatives in many applications.
Safety
As with any arsenic compound, handling arsenic trichloride requires extreme caution due to its toxicity. It can cause severe burns upon contact with skin and mucous membranes, and inhalation of its vapors can lead to respiratory irritation and damage.
Storage
It should be stored in tightly sealed containers in a well-ventilated area, away from heat, sparks, and open flames. Proper labeling and handling procedures should be followed to minimize the risk of exposure.
Due to its toxicity and potential health hazards, strict safety precautions must be observed when working with arsenic trichloride, including proper ventilation, protective clothing, and equipment. Disposal of arsenic-containing compounds must also be handled carefully to prevent environmental contamination and health risks.