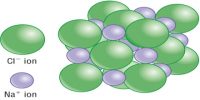
The absolute entropy of a pure substance at 25°C (298 K) and 1 atm pressure is called the standard entropy, S°. The standard entropies of all substances either elements or compounds at any temperature above 0°K always have positive values.
When the standard entropies, S° of various substances are known, the standard entropy change of a chemical process or reaction is written as
ΔS° = Σ S°products – Σ S°reactants
This ΔS° is the standard entropy change of the reaction.
Standard entropy change of formation, ΔS°f is defined as the entropy of formation of 1 mole of compound from the elements present in the standard conditions.ΔS°fcan be calculated for chemical compounds using the S° values of elements from which the compound is formed.
ΔS°f compound = ΣS°compound – Σ S°elements