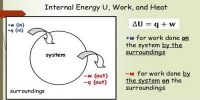
A thermodynamic state function which may be used to determine the direction of flow of heat from one thermal state to another and the function which remains constant in the adiabatic reversible process is said to be Entropy. In other words, the entropy is defined as the measure of unavailability of energy. This physical quantity is denoted by s.
The significance of entropy is:
- Entropy cannot be felt like temperature and pressure.
- It is a physical quantity.
- It indicates the direction of flow of heat.
- The orderliness of an object decreases with the increase of entropy.
- It helps in determining the thermodynamic state of an object.
- It expresses the state of a body like a temperature, pressure, volume, internal energy, magnetic behaviour.