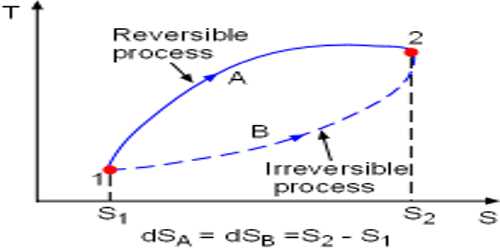
Reversible Process
Suppose, a piece of ice is in your hand. Keep this ice in a container and apply heat. It will be seen that by absorbing heat it has been transformed into water. Now keep the water in a deep freeze. You will see that water has been transformed into ice again. It is a reversible process.
So, it can be said that the process which reverses in opposite direction and in each stage of forwarding and reverse process the result of heat and work becomes equal and opposite, then that process is called reversible process.
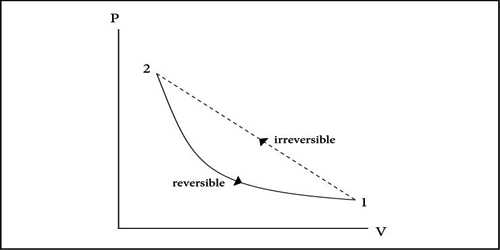
At normal pressure and at 273 K temperature if some amount of ice absorbs heat for transformation into the water and equal amount of water rejects the same amount of heat in order to be transformed into an ace, then it is a reversible process.
Characteristics
In the reversible process, the change of the system takes place extremely slowly and in a small amount during the whole change. The process takes place so slowly that every state maintains thermodynamical equilibrium. Besides, in this process, dissipative effects like inelasticity, viscosity, friction, electric resistance, magnetic hysteresis etc., will not exist. In short, it will be quasi-elastic and non-dissipative. This process is to be done in such a way that after the completion of the process the system and the surroundings can go back to the initial state without any net change. It is a very slow process and the system maintains thermal equilibrium.