Inter Molecular Attractive
All materials are composed of very tiny particles called atoms and molecules. Within these atoms and molecules there exist different types of attraction. Due to mutual attraction between molecules of the same material or molecules of different material surface tension is produced. But characteristics and nature of forces acting in different materials are not identical, differences exist.
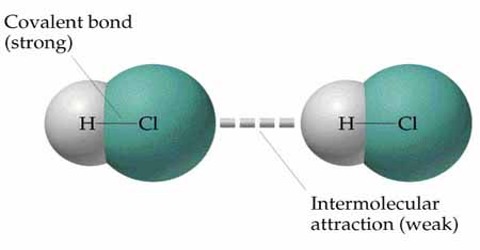
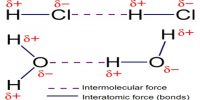
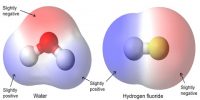
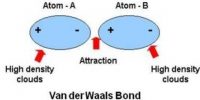
How molecules are attached for which solid, liquid and gaseous materials are formed? Actually molecules are attached to each other by a force. This force is called intermolecular force. That means, the force by which molecules are attached to each other to form different physical structures is called intermolecular force. From the following discussion we will be able to know the nature and characteristics of intermolecular force and repulsive force in case of solids, liquids and gases.
Basically there are 5 types of intermolecular forces.
- Ion-dipole interactions
- Dipole-dipole interactions
- Ion-induced interactions
- Dipole-induced interactions
- Induced-induced interactions/London forces/Dispersion forces
Among these we can’t directly say which is the strongest. It depends on the atoms connected with the bond (particularly the electro negativity). But we can generalize an order. But this is not always accurate.