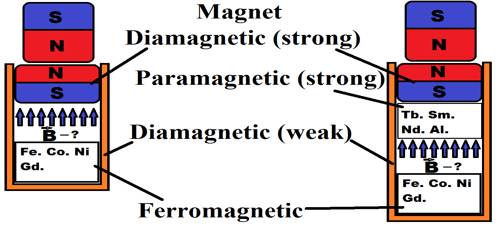
Diamagnetic substances are those in which the net magnetic moment of atoms is zero. There are no atomic dipoles in diamagnetic materials because the resultant magnetic moment of each atom is zero due to paired electrons. They are those substances which are feebly refeld by a magnet.
- The susceptibility has a low negative value. (For example, for bismuth Xm = – 0.00017).
- Susceptibility is independent of temperature. Magnetic susceptibility is small and negative.
- The relative permeability is slightly less than one. The relative permeability is slightly less than unity.
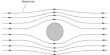
- When placed in a non-uniform magnetic field they have a tendency to move away from the field, (i.e) from the stronger part to the weaker part of the field. They get magnetized in a direction opposite to the field as shown in the Figure.
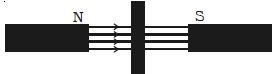
- When suspended freely in a uniform magnetic field, they set themselves perpendicular to the direction of the magnetic field (Figure).
These materials are independent of temperature, diamagnetic materials do not obey Curie’s law. Examples: Bi, Sb, Cu, Au, Hg, H2O, H2, etc.
Properties – Diamagnetic properties arise from the realignment of the electron paths under the influence of an external magnetic field.
- Diamagnetic materials have a weak, negative susceptibility to magnetic fields.
- When placed in a non-uniform magnetic field it moves from stronger to a weak field. When a diamagnetic substance is placed in a magnetic field it is weakly magnetized in the direction opposite to the including field.
- In diamagnetic materials all the electron are paired so there is no permanent net magnetic moment per atom.
- Most elements in the periodic table, including copper, silver, and gold, are diamagnetic.
- The substances are weakly repelled by the field so in a nonuniform field, these substances have a tendency to move from a strong to a weak part of the external magnetic field.
- A rod of diamagnetic material comes to rest with its length perpendicular to the direction of the field when it is suspended in a uniform magnetic field because the field is strongest at the poles. When a diamagnetic rod is freely suspended in a uniform magnetic field, it aligns itself in the direction itself perpendicular to the field.
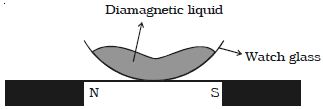
- If a diamagnetic liquid taken in a watch glass is placed in a uniform magnetic field, it collects away from the center when the magnetic poles are a loser and collect at the center.
- Induced dipole movement is a small negative value. The intensity of magnetization is has a small negative value.
- Magnetic permeability always less than unity (-ve). Magnetic susceptibility has a small negative value