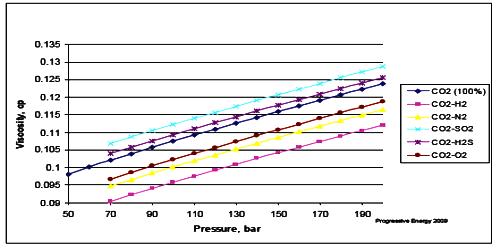
Effect of pressure on viscosity
We see that there is an effect of pressure on the coefficient of viscosity of liquids if pressure increases; the coefficient of viscosity also increases.
Explanation: As the pressure increases the intermolecular distance decreases; consequently, the intermolecular force increases. As a result, the relative velocity between two adjacent layers decreases, hence the coefficient of viscosity increases. For ideal gases, viscosity depends only on temperature. For real gases, that’s still a very good approximation. For liquids, changing the pressure does very little unless you increase the pressure a lot.
For most conditions near the circumstances we live in, pressure doesn’t have much effect on viscosity. For ideal gases, viscosity depends only on temperature. In most cases fluid viscosity increases with increasing pressure compared to the temperature influence, liquids persuade very less by the applied pressure. Informally, viscosity is the magnitude that describes a fluid’s conflict to flow. Fluids resist the virtual motion of absorbed objects from side to side them as well as to the motion of layers with conflicting velocities within them.
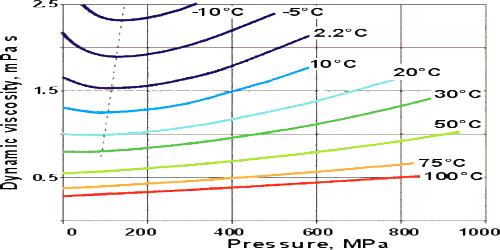
The viscosity of Liquids:
For most liquids, viscosity increases with rising pressure because the amount of free volume in the inside organization decreases due to compression.
The viscosity of Gases:
The viscosity of an ideal gas is self-sufficient of pressure, and this is approximately accurate for real gases. In gases, Viscosity arises mostly because of the transfer and exchange of molecular force.
Factors affecting viscosity –
Pressure has an effect on both, the viscosity of liquid as well as gases.
- On increasing pressure viscosity of liquid molecules increases due to the increase in the resistance to the flow of liquid.
- On increasing pressure, the viscosity of gas molecules decreases due to the increase in glow of molecules. Under most conditions, viscosity is independent of pressure. Viscosity has a pressure dependence for gases when the ideal gas model breaks down (e.g. at low temperatures and/or high pressures) and for liquids at very high pressure.
- Under most circumstances, viscosity is sovereign of pressure. Viscosity has a pressure dependence for gases when the ideal gas model breaks down (e.g. at low temperatures and/or high pressures) and for liquids at very high pressure.
- Viscosity is usually independent of pressure, but liquids under extreme pressure often experience an increase in viscosity. Since liquids are usually incompressible, an augment in pressure doesn’t actually bring the molecules appreciably closer together.
- The viscosity of gases increases as temperature increases and is around comparative to the square root of temperature. This is due to the augment in the incidence of intermolecular collisions at higher temperatures.
There is no effect of pressure on the coefficient of viscosity of gases. For most circumstances near the conditions we live in, pressure doesn’t have much effect on viscosity.