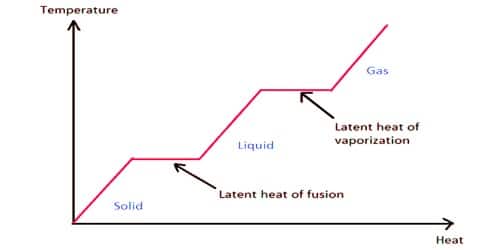
The energy required to change the phase of a substance is known as latent heat. The latent heat of fusion and vaporization both involve the heat required to change the state of a substance without a change in temperature. When the phase alters is from solid to liquid we must use the latent heat of fusion, and when the phase change is from liquid to a gas, we must use the latent heat of vaporization.
Latent heat of fusion: It is the process of change of state from solid to liquid.
We know, when the temperature of a solid reaches the melting point due to the application of heat then the temperature remains unchanged until all the substances transform into liquid. Here the amount of heat required to transform the solid into liquid is nothing but latent heat of fusion. The latent heat of fusion is the amount of heat required to change phase from solid to liquid at a constant temperature.
The latent heat of fusion is the enthalpy change of any measure of substance when it dissolves. This heat energy does not change the temperature but is used to loosen the intermolecular bond of the molecules of the substances. It is the heat consumed or discharged when matter melts, changing stage from strong to fluid-structure at a consistent temperature.
Latent heat of vaporization: It is the process of change of state from liquid to vapor.
The latent heat of vaporization is the amount of heat required to change phase from liquid to vapor at a constant temperature. If heat is applied to a liquid and its temperature reaches the boiling point than whatever amount of heat is applied the temperature will remain unchanged until all the liquid is converted into the vapor state. It is the heat consumed or discharged when matter disintegrates, changing the stage from fluid to gas stage at a consistent temperature. Here the amount of heat required to convert the liquid into vapor state is called the latent heat of vaporization. The heat of vaporization of water is the most elevated known.
Evaporation produces cooling: In summer days the water kept in a new earthen pitcher becomes cold. Uncountable numbers of pores are there on the body of an earthen pitcher. Through these pores, water seeps out and evaporates. The needed amount of latent heat is provided by the water of the pitcher and as such the water becomes cold. Note that latent heat is connected with no modification in temperature, yet a dissimilarity in the state. As a result of the high heat of vaporization, the vanishing of water has an articulated cooling impact and buildup has a warming contact.
Water kept in the glass or brass vessel does not become cold. Because the body of this kind of container has no pore and there is no possibility of evaporation.