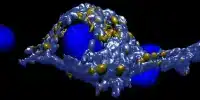
Zika virus is a type of virus that primarily infects human cells. Once the virus enters the human body, it targets specific cell types, such as skin cells, immune cells, and most importantly, certain cells of the nervous system.
Scientists solve the mystery of how Zika virus takes over key immune system cells. New findings shed light on how to stop the virus from spreading. Zika virus has a trick up its sleeve. Once inside the body, the virus likes to make a bee line for dendritic cells, the cells we rely on to launch an effective immune response.
“Dendritic cells are major cells of the innate immune system,” says LJI Professor Sujan Shresta, Ph.D., a member of the LJI Center for Infectious Disease and Vaccine Research. “How is this virus so clever that it’s able to establish infection in cells that would normally fight infections?”
Now Shresta and colleagues at LJI and the University of California, San Diego, have found that Zika virus actually forces dendritic cells to stop acting as immune cells. Using a new model of Zika virus infection, the LJI team showed that Zika virus instead makes dendritic cells churn out lipid molecules, which the virus uses to build copies of itself.
“Here are dendritic cells doing everything to help a virus,” says Shresta.
We demonstrated that Zika, but not dengue, modulates cellular metabolism to increase replication. These viruses are crazy. How these viruses manipulate host cell responses is very virus-specific and very cell-type specific.
Sujan Shresta
The Nature Communications study is a major step forward in the Shresta Lab’s work to guide the design of new antiviral therapies against many members of the Flavivirus family, including Zika, dengue, and Japanese encephalitis virus (JEV).
“Understanding how viruses interact with human cells is critical for understanding how to treat or prevent infection in the future,” says UC San Diego Professor Aaron Carlin, M.D., Ph.D., a former trainee in the Shresta Lab and co-leader of the new study.
Emilie Branche, Ph.D., a former postdoctoral researcher at LJI, led the effort to develop a model to better understand how Zika virus and dengue virus target dendritic cells. She worked with human immune cells called monocytes, which she prompted to differentiate into dendritic cells.
Branche analyzed how gene expression in these dendritic cells shifted during a Zika or dengue infection. She then compared the changes in gene expression with changes in cells she subjected to a “mock infection.” This comparison revealed precisely how Zika pulls off its cellular take-over.
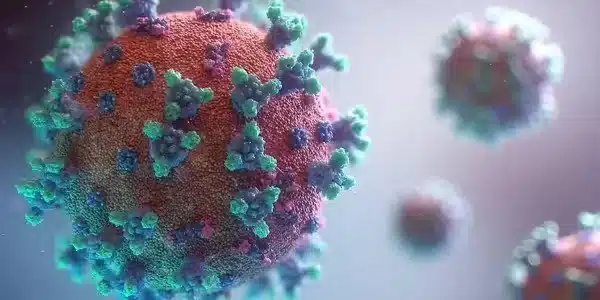
The Zika virus, according to the researchers, manipulates the genes that control lipid metabolism in dendritic cells. The virus activates a cellular protein called SREBP, which causes lipid, or fat molecule, production to ramp up. These lipids were used to assemble new copies of the Zika virus, which were designed to spread throughout the body and cause further infection.
“We demonstrated that Zika, but not dengue, modulates cellular metabolism to increase replication,” Branche says. The researchers then looked into whether Zika transforms other cells into lipid factories. Despite the fact that Zika is known to target neuronal precursor cells, the researchers demonstrated that Zika does not alter the lipid metabolism genes in those cells. Shresta was surprised to see these changes only in dendritic cells, and she was surprised that Zika, not dengue, altered lipid production.
“These viruses are crazy,” says Shresta. “How these viruses manipulate host cell responses is very virus-specific and very cell-type specific.”
The next step is to develop antivirals that stop Zika from exploiting the genes for lipid metabolism. The new study shows that therapeutically silencing SREBP may hold promise. What to do about Zika’s cousin, dengue? Because these viruses are so closely related and overlap in so many places, Shresta imagines a SREBP inhibitor as just one ingredient in a “cocktail” of inhibitors to treat many different flavivirus infections.
“The more knowledge we can generate about these viruses, the closer we are to a ‘pan-flavivirus’ inhibitor,” she says.