Cancer is expected to claim the lives of 10 million people by 2020. Changes to our DNA, which serves as the instruction manual for all of our cells, are at the root of this devastating disease. It has been 20 years since scientists first revealed the human genome sequence. This historic achievement was followed by significant technological advances that now allow us to read the layers of information in our DNA in incredible detail, from the first changes in DNA that occur when a cell becomes cancerous to the complex microenvironments of advanced tumors.
Now, in order to speed up cancer research, we need new ways to bring together the various types of complex data that we generate in order to provide new biological insights into cancer evolution.
For today’s issue of Science, my colleagues Professor Toshikazu Ushijima, Chief, Epigenomics Division, National Cancer Center Research Institute (Japan), Prof Patrick Tan, Executive Director, Genome Institute of Singapore, and I were invited to review the cancer insights we can currently obtain from analyzing DNA in its entirety, as well as define the future challenges we must face to yield the results we seek.
In 2020, an estimated 10 million people lost their lives to cancer. This devastating disease is underpinned by changes to our DNA — the instruction manual for all our cells.
The complexity of our DNA
Many people think of our DNA, or genome, as a series of letters. In reality, many layers of information, referred to as the epigenome, completely alter its activity. Our genome can be compared to our planet’s various geographical environments. Our genetic sequence of As, Ts, Gs, and Cs forms the basis of complex structural features within our cells, much like mountains, islands, and oceans are made up of the same basic elements.
Our epigenome creates these geographical environments, which include chemical markers that attach to our DNA (called DNA methylation) and chemical changes to proteins (histones) that wrap around it, which together orchestrate how DNA is organized in three dimensions inside our cells.
During the cancer life cycle, both our genome and epigenome evolve, and we need to understand these complex changes to improve cancer risk assessment and accelerate therapeutic discoveries for patients.
From cancer formation to metastasis
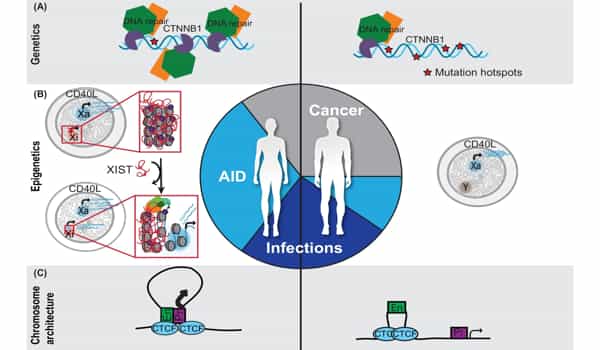
It was previously thought that only genetic changes could cause cancer, but it is now clear that both genome and epigenome changes play a significant role in cancer evolution. There is some evidence that changes in DNA methylation that occur with aging, for example, may predispose cells to genetic changes that cause cancer.
Take, for example, cigarette smoking, where scientists have discovered DNA methylation changes in the cells lining the lung long before genetic changes or lung cancer can be detected. We need to map the precise order of genomic and epigenomic changes to gain new insights into what drives carcinogenesis.
We are also learning that, while cancer can accumulate genetic changes, the epigenome is also ‘reprogrammed’ as cancer progresses from a primary to a metastasizing tumor and eventually develops treatment resistance. Understanding these changes may lead to new therapeutic targets that can be used to treat advanced cancers more precisely.
New insight through advanced technologies
Cancer cells coexist in a tumor ecosystem with other cell types such as immune cells and connective cells known as stromal cells. Today, advanced imaging and single-cell technologies are assisting us in mapping these cells, as well as genomic and epigenomic changes, in the three-dimensional context of a tumor with unprecedented resolution. These studies are being carried out at Garvan by our researchers using our intravital microscopy facilities and the Garvan-Weizmann Centre for Cellular Genomics.
To study cancers at the single-cell and spatial levels, a number of international research consortia have been formed, including the Human Tumour Atlas Network and the Cancer Research UK Grand Challenge project. These consortia, however, will face enormous challenges in data integration. In today’s global research environment, globally standardized methods for integrating data from various analysis techniques and laboratories are required.
We will understand how cancer can be better diagnosed, treated, and prevented by revealing not just associations, but the full integration of DNA and cellular changes that occur during cancer formation and progression.
Big data – opportunities and challenges
Over the last two decades, we’ve developed the technology to demonstrate that our genome and epigenome are far more complex than we previously thought. We’ve arrived at the point where new cancer insights will be gained by solving mathematical problems generated by complex and diverse sequencing and imagining data sets.
Our advanced technologies enable us to generate massive amounts of data. However, the current challenge is data integration; humans simply cannot digest all of the information we generate. This challenge will be addressed by artificial intelligence, which will require us to incorporate computational expertise in order to look at and model data in novel ways.
Another critical future challenge will be translating basic research findings into practical clinical applications. A precise understanding of the multiple steps that lead to cancer formation inside cells may enable us to improve cancer risk screening and early detection. In the future, research into genetic and epigenetic signatures may aid in the elimination of carcinogenic agents and processes from our environment.
For advanced cancers, integrated DNA analyses may aid in identifying previously unknown mechanisms by which cancer cells metastasize, which may be promising targets for therapy development. The challenge of integrating our data to study cancer as geneticists and epigeneticists is similar to the challenge of modeling climate change. Climate modeling necessitates the collection and contextualization of massive amounts of data from various sources in order to make predictions about the planet’s future.
This is also true for genomics and epigenomics: we need to understand how the various layers of DNA information interact to cause the damaging effects of “climate change” in our cells as they become cancerous.