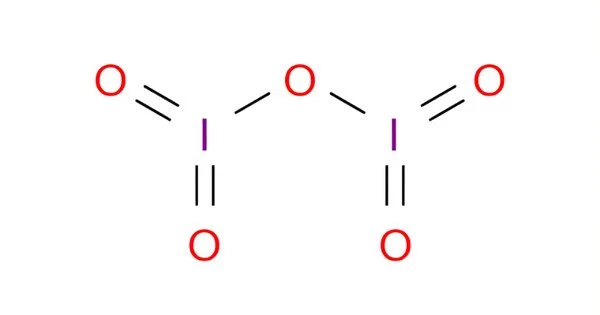
Iodine pentoxide is a chemical compound with the formula I2O5. It is an oxide of iodine and is considered an interhalogen compound. This iodine oxide is the anhydride of iodic acid and the only stable oxide of iodine. The molecular formula indicates that it contains two atoms of iodine and five atoms of oxygen. It is produced by dehydrating iodic acid at 200 °C in a stream of dry air:
2HIO3 → I2O5 + H2O
Properties
- Chemical formula: I2O5
- Molar mass: 333.81 g/mol
- Appearance: white crystalline solid hygroscopic
- Density: 4.980 g/cm3
- Melting point: 300 °C (572 °F; 573 K)[2] (decomposes)
- Solubility: soluble in water and nitric acid; insoluble in ethanol, ether, and CS2
Structure
I2O5 is bent with an I–O–I angle of 139.2°, but the molecule has no mirror plane so its symmetry is C2 rather than C2v. The terminal I–O distances are around 1.80 Å and the bridging I–O distances are around 1.95 Å.
The structure of iodine pentoxide is often represented as I₄O₁₀, which reflects its tetrameric structure. Each iodine atom is bonded to two oxygen atoms, and there are bridging oxygen atoms between the iodine atoms. The compound has a molecular weight of approximately 333.71 g/mol.
Reactions
Iodine pentoxide is a powerful oxidizing agent and can react violently with reducing agents. It is not commonly encountered in everyday life and is mostly used in laboratory settings for specific chemical reactions. Due to its reactivity and potential hazards, it requires careful handling.
Iodine pentoxide easily oxidizes carbon monoxide to carbon dioxide at room temperature:
5 CO + I2O5 → I2 + 5 CO2
This reaction can be used to analyze the concentration of CO in a gaseous sample. I2O5 forms iodyl salts, [IO2+], with SO3 and S2O6F2, but iodosyl salts, [IO+], with concentrated sulfuric acid. Iodine pentoxide decomposes to iodine (vapor) and oxygen when heated to about 350 °C.
Applications
- Chemical Synthesis: It is used in chemical laboratories for the synthesis of various organic compounds. It can act as an oxidizing agent in certain reactions.
- Dehydrating Agent: It is a strong dehydrating agent, meaning it can remove water molecules from substances. It is used in dehydration reactions in organic chemistry.
- Rocket Propellant: It has been considered as an ingredient in rocket propellants. However, due to its reactivity and potential hazards, other, less reactive oxidizers are often preferred.