Blood clotting, also known as coagulation, is a complex biological process that aids in wound healing by preventing excessive bleeding after an injury. It entails a number of steps as well as the interaction of various molecules and cells. Blood platelet glycoprotein V is a critical switch for haemostasis and thrombus formation. This new discovery could have significant clinical implications.
When our blood vessels are injured by cuts, abrasions, or bruises, it is critical to stop the bleeding and seal the wound. This is known as hemostasis, and it consists of two major components: First, blood platelets adhere to the wound edges, forming a plug that temporarily seals the injury. Second, blood coagulation or the coagulation cascade is initiated, resulting in the formation of long fibrin fibers that, along with platelets, tightly seal the wound.
However, thrombosis and subsequent vascular occlusions can occur if fibrin is formed in excess, as in chronic wounds. As a result, it is critical to strictly regulate fibrin formation. Until now, it was unclear how blood clotting is limited.
In an international project coordinated by the University Hospital of Würzburg, researchers have now deciphered a central regulatory mechanism of fibrin formation and propose new therapeutic approaches. The results have been published in the journal Nature Cardiovascular Research.
We were able to demonstrate in our studies that these antibodies increase thrombin activity, resulting in increased fibrin formation. Our idea was to use these antibodies to increase fibrin formation in the context of impaired hemostasis.
Professor David Stegner
GPV controls thrombin activity and fibrin formation
Professor Bernhard Nieswandt’s research group discovered a fundamentally new mechanism in this study:
“For the first time, we were able to uncover a new switching point that regulates both hemostasis and thrombosis. This switch is glycoprotein V, also known as GPV, which is found on the surface of blood platelets. GPV regulates the activity of the enzyme thrombin, which is responsible for fibrin formation,” explains Bernhard Nieswandt, head of the Institute of Experimental Biomedicine I and member of the Rudolf Virchow Centre – Centre for Integrative and Translational Bioimaging (RVZ) at the University of Würzburg.
Thrombin is a critical enzyme in blood clotting, so its activity must be precisely spatiotemporally controlled. Until now, it was thought that thrombin cleaves the surface receptor GPV during platelet activation. This results in the release of GPV in a soluble form. However, the physiological function of this receptor was largely unknown. Using genetic and pharmacological approaches, the researchers demonstrated that thrombin-mediated cleavage of GPV limits fibrin formation. Soluble GPV alters the activity of thrombin by remaining bound to it, allowing it to form less fibrin.
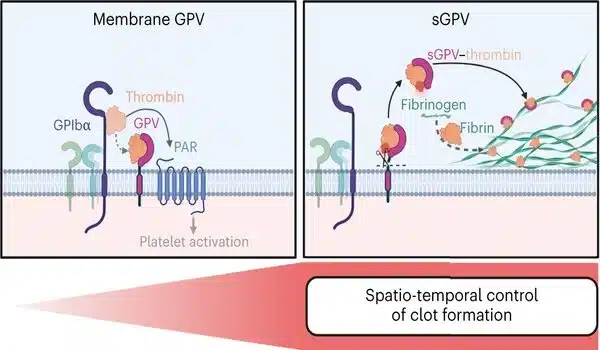
“Findings will change textbook knowledge”
In experimental thrombosis models, soluble GPV was shown to prevent, among other things, the formation of vaso-occlusive thrombi and to lead to a significant protection from experimental stroke and associated brain damage.
Bernhard Nieswandt is convinced that these new findings will change textbook knowledge. He thanks all participating scientists from the RVZ and the University Hospital Würzburg (UKW), who were supported by colleagues from Mainz, Maastricht, and the USA.
Antibodies against GPV offer great clinical potential in the treatment of disturbed hemostasis
In another approach, the researchers created antibodies against GPV that prevent the virus from being cleaved by thrombin.
“We were able to demonstrate in our studies that these antibodies increase thrombin activity, resulting in increased fibrin formation. Our idea was to use these antibodies to increase fibrin formation in the context of impaired hemostasis,” says Professor David Stegner, head of the RVZ Vascular Imaging Group and one of the study’s final authors.
A reduced platelet count or impaired function can be caused by pharmacological treatment, in addition to genetic causes, and thus lead to impaired hemostasis. Platelet aggregation inhibitors, such as clopidogrel, which are used to prevent heart attacks and strokes and to treat circulatory disorders, such as atrial fibrillation, impair platelet function.
“Our new antibody was able to restore hemostasis in an experimental model of hemostasis under conditions where hemostasis would otherwise be impossible.” Dr. Sarah Beck, scientist at the Würzburg Institute for Experimental Biomedicine and the study’s first author, adds, “This indicates support of hemostasis by enhancing thrombin-dependent fibrin formation. Anti-GPV treatment has a lot of clinical potential.” This will be looked into further in the future.”