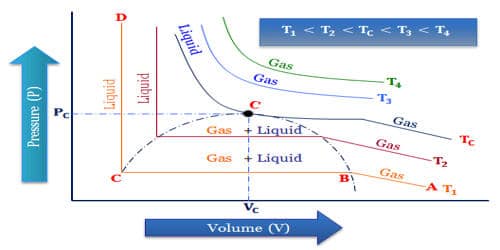
Critical Temperature is an important term for the liquefaction of gases. The liquefaction of gas takes place when the intermolecular forces of attraction become so high that they bind the gas molecules together to form the liquid state. We know the critical temperature is the temperature above which the translational energy of the molecules is so high so that intermolecular attraction does not become sufficient for liquefying the gases and pressure cannot play any role to come to contact of the molecules. So for liquefying a gas its temperature must remain at critical temperature or below the critical temperature. Then it can be easily liquefied by applying pressure.
The critical temperature of a gas is a measure of the strength of the intermolecular forces of attraction. The intermolecular forces of attraction can be increased either by increasing the pressure so that the molecules come close together or by cooling the gas so that the kinetic energy of the molecules decreases and they become slower. Weaker are the intermolecular forces, more difficult it is to liquefy that gas and hence lower would be the critical temperature of that gas. The effect of temperature on the liquefaction of gases is found to be very important as higher the temperature of the gas, more difficult it is to liquefy it and higher is the pressure required. The critical temperature of a gas may be defined as that temperature above which it cannot be liquefied however high pressure may be applied to the gas. The pressure required to liquefy the gas at the critical temperature is called critical pressure.
Differences in critical temperatures among gases mean that some gases are easier to liquify than are others. Helium and hydrogen have weak intermolecular forces, thus they are difficult to liquefy and hence have a low critical temperature. By comparison, the critical temperature of nitrogen gas is 126K (-232.6°F [-147°C]) and that of helium is 5.3K (-449.9°F [-267.7°C]). The critical temperature of carbon dioxide is high enough so that it can be liquified relatively easily at or near room temperature. Carbon dioxide and ammonia have strong intramolecular forces of attraction, they can be easily liquefied and their critical temperature is high which are above room temperature. Liquefying gases such as nitrogen and helium obviously present much greater difficulties than does the liquefaction of carbon dioxide. For example, Carbon dioxide below its critical temperature is called carbon dioxide vapor.